Synchronous and asynchronous transmitter release at nicotinic synapses are differentially regulated by postsynaptic PSD-95 proteins
- PMID: 20016093
- PMCID: PMC2844112
- DOI: 10.1523/JNEUROSCI.4951-09.2009
Synchronous and asynchronous transmitter release at nicotinic synapses are differentially regulated by postsynaptic PSD-95 proteins
Abstract
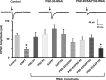
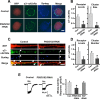
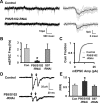
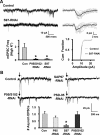
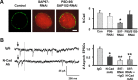
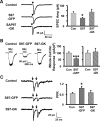
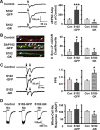
The rate and timing of information transfer at neuronal synapses are critical for determining synaptic efficacy and higher network function. Both synchronous and asynchronous neurotransmitter release shape the pattern of synaptic influences on a neuron. The PSD-95 family of postsynaptic scaffolding proteins, in addition to organizing postsynaptic components at glutamate synapses, acts transcellularly to regulate synchronous glutamate release. Here we show that PSD-95 family members at nicotinic synapses on chick ciliary ganglion neurons in culture execute multiple functions to enhance transmission. Together, endogenous PSD-95 and SAP102 in the postsynaptic cell appear to regulate transcellularly the synchronous release of transmitter from presynaptic terminals onto the neuron while stabilizing postsynaptic nicotinic receptor clusters under the release sites. Endogenous SAP97, in contrast, has no effect on receptor clusters but acts transcellularly from the postsynaptic cell through N-cadherin to enhance asynchronous release. These separate and parallel regulatory pathways allow postsynaptic scaffold proteins to dictate the pattern of cholinergic input a neuron receives; they also require balancing of PSD-95 protein levels to avoid disruptive competition that can occur through common binding domains.
Figures
Similar articles
-
Capabilities of neurexins in the chick ciliary ganglion.Dev Neurobiol. 2008 Feb 15;68(3):409-19. doi: 10.1002/dneu.20598. Dev Neurobiol. 2008. PMID: 18161851
-
Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein.J Neurosci. 2004 Jul 28;24(30):6776-84. doi: 10.1523/JNEUROSCI.1826-04.2004. J Neurosci. 2004. PMID: 15282282 Free PMC article.
-
The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo.J Neurosci. 2010 Aug 18;30(33):11073-85. doi: 10.1523/JNEUROSCI.0983-10.2010. J Neurosci. 2010. PMID: 20720115 Free PMC article.
-
The postsynaptic organization of synapses.Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a005678. doi: 10.1101/cshperspect.a005678. Cold Spring Harb Perspect Biol. 2011. PMID: 22046028 Free PMC article. Review.
-
MAGUKs, synaptic development, and synaptic plasticity.Neuroscientist. 2011 Oct;17(5):493-512. doi: 10.1177/1073858410386384. Epub 2011 Apr 15. Neuroscientist. 2011. PMID: 21498811 Free PMC article. Review.
Cited by
-
Bayesian analysis of the kinetics of quantal transmitter secretion at the neuromuscular junction.J Comput Neurosci. 2015 Oct;39(2):119-29. doi: 10.1007/s10827-015-0567-3. Epub 2015 Jul 2. J Comput Neurosci. 2015. PMID: 26129670
-
Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type.J Neurosci. 2010 Jun 30;30(26):8841-51. doi: 10.1523/JNEUROSCI.6236-09.2010. J Neurosci. 2010. PMID: 20592206 Free PMC article.
-
Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release.Annu Rev Physiol. 2014;76:333-63. doi: 10.1146/annurev-physiol-021113-170338. Epub 2013 Nov 21. Annu Rev Physiol. 2014. PMID: 24274737 Free PMC article. Review.
-
PMCA2 via PSD-95 controls calcium signaling by α7-containing nicotinic acetylcholine receptors on aspiny interneurons.J Neurosci. 2012 May 16;32(20):6894-905. doi: 10.1523/JNEUROSCI.5972-11.2012. J Neurosci. 2012. PMID: 22593058 Free PMC article.
-
N-cadherin induces partial differentiation of cholinergic presynaptic terminals in heterologous cultures of brainstem neurons and CHO cells.Front Synaptic Neurosci. 2012 Dec 5;4:6. doi: 10.3389/fnsyn.2012.00006. eCollection 2012. Front Synaptic Neurosci. 2012. PMID: 23227006 Free PMC article.
References
-
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. - PubMed
-
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
-
- Brusés JL. N-Cadherin signaling in synapse formation and neuronal physiology. Mol Neurobiol. 2006;33:237–252. - PubMed
-
- Chen M, Pugh PC, Margiotta JF. Nicotinic synapses formed between chick ciliary ganglion neurons in culture resemble those present on the neurons in vivo. J Neurobiol. 2001;47:265–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
