Cooperative and competitive interactions facilitate stereo computations in macaque primary visual cortex
- PMID: 20016094
- PMCID: PMC5788183
- DOI: 10.1523/JNEUROSCI.2305-09.2009
Cooperative and competitive interactions facilitate stereo computations in macaque primary visual cortex
Abstract
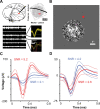
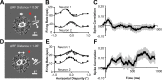
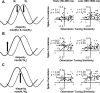
Inferring depth from binocular disparities is a difficult problem for the visual system because local features in the left- and right-eye images must be matched correctly to solve this "stereo correspondence problem." Cortical architecture and computational studies suggest that lateral interactions among neurons could help resolve local uncertainty about disparity encoded in individual neurons by incorporating contextual constraints. We found that correlated activity among pairs of neurons in primary visual cortex depended both on disparity-tuning relationships and the stimuli displayed within the receptive fields of the neurons. Nearby pairs of neurons with distinct disparity tuning exhibited a decrease in spike correlation at competing disparities soon after response onset. Distant neuronal pairs of similar disparity tuning exhibited an increase in spike correlation at mutually preferred disparities. The observed correlated activity and response dynamics suggests that local competitive and distant cooperative interactions improve disparity tuning of individual neurons over time. Such interactions could represent a neural substrate for the principal constraints underlying cooperative stereo algorithms.
Figures











Similar articles
-
Binocular spatial phase tuning characteristics of neurons in the macaque striate cortex.J Neurophysiol. 1997 Jul;78(1):351-65. doi: 10.1152/jn.1997.78.1.351. J Neurophysiol. 1997. PMID: 9242285
-
Evidence of Stereoscopic Surface Disambiguation in the Responses of V1 Neurons.Cereb Cortex. 2017 Mar 1;27(3):2260-2275. doi: 10.1093/cercor/bhw064. Cereb Cortex. 2017. PMID: 26965904 Free PMC article.
-
Disparity-tuning characteristics of neuronal responses to dynamic random-dot stereograms in macaque visual area V4.J Neurophysiol. 2005 Oct;94(4):2683-99. doi: 10.1152/jn.00319.2005. Epub 2005 Jul 6. J Neurophysiol. 2005. PMID: 16000525
-
Mechanisms of stereopsis in monkey visual cortex.Cereb Cortex. 1995 May-Jun;5(3):193-204. doi: 10.1093/cercor/5.3.193. Cereb Cortex. 1995. PMID: 7613075 Review.
-
Human cortical areas underlying the perception of optic flow: brain imaging studies.Int Rev Neurobiol. 2000;44:269-92. doi: 10.1016/s0074-7742(08)60746-1. Int Rev Neurobiol. 2000. PMID: 10605650 Review.
Cited by
-
Sample skewness as a statistical measurement of neuronal tuning sharpness.Neural Comput. 2014 May;26(5):860-906. doi: 10.1162/NECO_a_00582. Epub 2014 Feb 20. Neural Comput. 2014. PMID: 24555451 Free PMC article.
-
Population activity structure of excitatory and inhibitory neurons.PLoS One. 2017 Aug 17;12(8):e0181773. doi: 10.1371/journal.pone.0181773. eCollection 2017. PLoS One. 2017. PMID: 28817581 Free PMC article.
-
Neural dynamics of image representation in the primary visual cortex.J Physiol Paris. 2012 Sep-Dec;106(5-6):250-65. doi: 10.1016/j.jphysparis.2012.08.006. Epub 2012 Aug 31. J Physiol Paris. 2012. PMID: 22944076 Free PMC article.
-
Dynamic mechanisms of visually guided 3D motion tracking.J Neurophysiol. 2017 Sep 1;118(3):1515-1531. doi: 10.1152/jn.00831.2016. Epub 2017 Jun 21. J Neurophysiol. 2017. PMID: 28637820 Free PMC article.
-
Correlated discharges in the primate prefrontal cortex before and after working memory training.Eur J Neurosci. 2012 Dec;36(11):3538-48. doi: 10.1111/j.1460-9568.2012.08267.x. Epub 2012 Aug 30. Eur J Neurosci. 2012. PMID: 22934919 Free PMC article.
References
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 2003;97:141–154. - PubMed
-
- Ben-Shahar O, Huggins PS, Izo T, Zucker SW. Cortical connections and early visual function: intra- and inter-columnar processing. J Physiol Paris. 2003;97:191–208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
