Neuroprotective role of haptoglobin after intracerebral hemorrhage
- PMID: 20016097
- PMCID: PMC2831226
- DOI: 10.1523/JNEUROSCI.3776-09.2009
Neuroprotective role of haptoglobin after intracerebral hemorrhage
Abstract
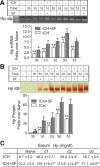
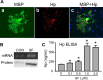
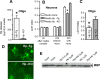
After intracerebral hemorrhage (ICH), the brain parenchyma is exposed to blood containing red blood cells (RBCs) and consequently to its lysis products. Iron-rich hemoglobin (Hb) is the most abundant protein in RBCs. When released into the brain parenchyma during hemolysis, Hb becomes a central mediator of cytotoxicity. Our study indicates that haptoglobin (Hp), an acute-phase response protein primarily synthesized in the liver and known to bind and neutralize Hb in the bloodstream, is also expressed in brain in which it plays an important role in defending neurons from damage induced by hemolytic products after ICH. We demonstrate that the Hb-induced hypohaptoglobinemia aggravates ICH-induced brain damage while pharmacologic intervention with sulforaphane to induce brain Hp is linked to a reduction in brain damage. In agreement with these findings, Hp deficiency worsens whereas Hp overexpression alleviates ICH-mediated brain injury. We also identified that oligodendroglia are the primary source of brain-derived Hp among brain cells and that oligodendroglia-released Hp plays protective roles against Hb-mediated toxicity to neurons and oligodendrocytes. We conclude that Hp, particularly the brain-derived Hp, plays cytoprotective roles and represents a potential therapeutic target for ICH treatment.
Figures




References
-
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. - PubMed
-
- Baumann H, Muller-Eberhard U. Synthesis of hemopexin and cysteine protease inhibitor is coordinately regulated by HSF-II and interferon-beta 2 in rat hepatoma cells. Biochem Biophys Res Commun. 1987;146:1218–1228. - PubMed
-
- Bhasin RR, Xi G, Hua Y, Keep RF, Hoff JT. Experimental intracerebral hemorrhage: effect of lysed erythrocytes on brain edema and blood-brain barrier permeability. Acta Neurochir Suppl. 2002;81:249–251. - PubMed
-
- Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006;66:634–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
