Endolymphatic sodium homeostasis by extramacular epithelium of the saccule
- PMID: 20016101
- PMCID: PMC3849662
- DOI: 10.1523/JNEUROSCI.3044-09.2009
Endolymphatic sodium homeostasis by extramacular epithelium of the saccule
Abstract
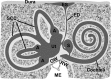
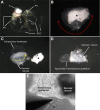
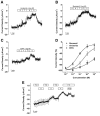
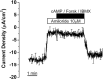
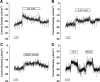
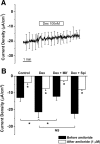
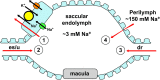
The saccule is a vestibular sensory organ that depends upon regulation of its luminal fluid, endolymph, for normal transduction of linear acceleration into afferent neural transmission. Previous studies suggested that endolymph in the saccule was merely derived from cochlear endolymph. We developed and used a preparation of isolated mouse saccule to measure transepithelial currents from the extramacular epithelium with a current density probe. The direction and pharmacology of transepithelial current was consistent with Na(+) absorption by the epithelial Na(+) channel (ENaC) and was blocked by the ENaC-specific inhibitors benzamil and amiloride. Involvement of Na(+),K(+)-ATPase and K(+) channels was demonstrated by reduction of the current by ouabain and the K(+) channel blockers Ba(2+), XE991, and 4-AP. Glucocorticoids upregulated the current via glucocorticoid receptors. Dexamethasone stimulated the current after 24 h and the stimulation was blocked by mifepristone but not spironolactone. No acute response was observed to elevated cAMP in the presence of amiloride nor to bumetanide, a blocker of Na(+),K(+),2Cl(-) cotransporter. The results are consistent with a canonical model of corticosteroid-regulated Na(+) absorption that includes entry of luminal Na(+) through apical membrane Na(+) channels and active basolateral exit of Na(+) via a Na(+) pump, with recycling of K(+) at the basolateral membrane via K(+)-permeable channels. These observations provide our first understanding of the active role played by saccular epithelium in the local regulation of the [Na(+)] of endolymph for maintenance of our sense of balance.
Figures

References
-
- Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. 1987;5:202–207. - PubMed
-
- Barrs DM, Keyser JS, Stallworth C, McElveen JT., Jr Intratympanic steroid injections for intractable Meniere's disease. Laryngoscope. 2001;111:2100–2104. - PubMed
-
- Bhargava A, Pearce D. Mechanisms of mineralocorticoid action: determinants of receptor specificity and actions of regulated gene products. Trends Endocrinol Metab. 2004;15:147–153. - PubMed
-
- Böhmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A, Lin JT, Lang F, Wehner F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem. 2000;10:187–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
