An intrinsic neuronal oscillator underlies dopaminergic neuron bursting
- PMID: 20016105
- PMCID: PMC2824818
- DOI: 10.1523/JNEUROSCI.4053-09.2009
An intrinsic neuronal oscillator underlies dopaminergic neuron bursting
Abstract
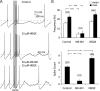
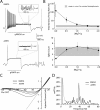
Dopaminergic neurons of the ventral midbrain fire high-frequency bursts when animals are presented with unexpected rewards, or stimuli that predict reward. To identify the afferents that can initiate bursting and establish therapeutic strategies for diseases affected by altered bursting, a mechanistic understanding of bursting is essential. Our results show that bursting is initiated by a specific interaction between the voltage sensitivity of NMDA receptors and voltage-gated ion channels that results in the activation of an intrinsic, action potential-independent, high-frequency membrane potential oscillation. We further show that the NMDA receptor is uniquely suited for this because of the rapid kinetics and voltage dependence imparted to it by Mg(2+) ion block and unblock. This mechanism explains the discrete nature of bursting in dopaminergic cells and demonstrates how synaptic signals may be reshaped by local intrinsic properties of a neuron before influencing action potential generation.
Figures





References
-
- Amini B, Clark JW, Jr, Canavier CC. Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J Neurophysiol. 1999;82:2249–2261. - PubMed
-
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J Neurophysiol. 2007;97:2837–2850. - PubMed
-
- Canavier CC. Sodium dynamics underlying burst firing and putative mechanisms for the regulation of the firing pattern in midbrain dopamine neurons: a computational approach. J Comput Neurosci. 1999;6:49–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
