Auditory cortical activity after intracortical microstimulation and its role for sensory processing and learning
- PMID: 20016106
- PMCID: PMC6666178
- DOI: 10.1523/JNEUROSCI.1949-09.2009
Auditory cortical activity after intracortical microstimulation and its role for sensory processing and learning
Abstract
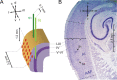
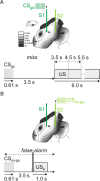
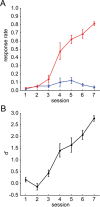
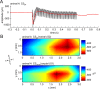
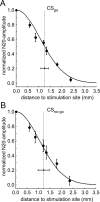
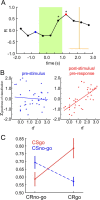
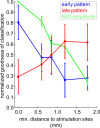
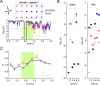
Several studies have shown that animals can learn to make specific use of intracortical microstimulation (ICMS) of sensory cortex within behavioral tasks. Here, we investigate how the focal, artificial activation by ICMS leads to a meaningful, behaviorally interpretable signal. In natural learning, this involves large-scale activity patterns in widespread brain-networks. We therefore trained gerbils to discriminate closely neighboring ICMS sites within primary auditory cortex producing evoked responses largely overlapping in space. In parallel, during training, we recorded electrocorticograms (ECoGs) at high spatial resolution. Applying a multivariate classification procedure, we identified late spatial patterns that emerged with discrimination learning from the ongoing poststimulus ECoG. These patterns contained information about the preceding conditioned stimulus, and were associated with a subsequent correct behavioral response by the animal. Thereby, relevant pattern information was mainly carried by neuron populations outside the range of the lateral spatial spread of ICMS-evoked cortical activation (approximately 1.2 mm). This demonstrates that the stimulated cortical area not only encoded information about the stimulation sites by its focal, stimulus-driven activation, but also provided meaningful signals in its ongoing activity related to the interpretation of ICMS learned by the animal. This involved the stimulated area as a whole, and apparently required large-scale integration in the brain. However, ICMS locally interfered with the ongoing cortical dynamics by suppressing pattern formation near the stimulation sites. The interaction between ICMS and ongoing cortical activity has several implications for the design of ICMS protocols and cortical neuroprostheses, since the meaningful interpretation of ICMS depends on this interaction.
Figures


Similar articles
-
Intracortical Microstimulation Modulates Cortical Induced Responses.J Neurosci. 2018 Sep 5;38(36):7774-7786. doi: 10.1523/JNEUROSCI.0928-18.2018. Epub 2018 Jul 27. J Neurosci. 2018. PMID: 30054394 Free PMC article.
-
Cathodic-leading pulses are more effective than anodic-leading pulses in intracortical microstimulation of the auditory cortex.J Neural Eng. 2019 Jun;16(3):036002. doi: 10.1088/1741-2552/ab0944. Epub 2019 Feb 21. J Neural Eng. 2019. PMID: 30790776
-
Intracortical microstimulation induced changes in spectral and temporal response properties in cat auditory cortex.Hear Res. 2003 Sep;183(1-2):109-25. doi: 10.1016/s0378-5955(03)00220-x. Hear Res. 2003. PMID: 13679143
-
Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli.J Neurosci. 2014 Jan 22;34(4):1234-47. doi: 10.1523/JNEUROSCI.1990-13.2014. J Neurosci. 2014. PMID: 24453315 Free PMC article.
-
Neural hijacking: action of high-frequency electrical stimulation on cortical circuits.Neuroscientist. 2013 Oct;19(5):434-41. doi: 10.1177/1073858412458368. Epub 2012 Sep 10. Neuroscientist. 2013. PMID: 22968640 Free PMC article. Review.
Cited by
-
Laser-Induced Apoptosis of Corticothalamic Neurons in Layer VI of Auditory Cortex Impact on Cortical Frequency Processing.Front Neural Circuits. 2021 Jul 12;15:659280. doi: 10.3389/fncir.2021.659280. eCollection 2021. Front Neural Circuits. 2021. PMID: 34322001 Free PMC article.
-
A statistical method for analyzing and comparing spatiotemporal cortical activation patterns.Sci Rep. 2018 Apr 3;8(1):5433. doi: 10.1038/s41598-018-23765-w. Sci Rep. 2018. PMID: 29615797 Free PMC article.
-
Large-scale heterogeneous representation of sound attributes in rat primary auditory cortex: from unit activity to population dynamics.J Neurosci. 2011 Oct 12;31(41):14639-53. doi: 10.1523/JNEUROSCI.0086-11.2011. J Neurosci. 2011. PMID: 21994380 Free PMC article.
-
Discriminability of Single and Multichannel Intracortical Microstimulation within Somatosensory Cortex.Front Bioeng Biotechnol. 2016 Dec 2;4:91. doi: 10.3389/fbioe.2016.00091. eCollection 2016. Front Bioeng Biotechnol. 2016. PMID: 27995126 Free PMC article.
-
Behavioral and electrophysiological effects of cortical microstimulation parameters.PLoS One. 2013 Dec 5;8(12):e82170. doi: 10.1371/journal.pone.0082170. eCollection 2013. PLoS One. 2013. PMID: 24340002 Free PMC article.
References
-
- Barrie JM, Freeman WJ, Lenhart MD. Spatiotemporal analysis of prepyriform, visual, auditory, and somesthetic surface EEGs in trained rabbits. J Neurophysiol. 1996;76:520–539. - PubMed
-
- Bartlett JR, DeYoe EA, Doty RW, Lee BB, Lewine JD, Negrão N, Overman WH., Jr Psychophysics of electrical stimulation of striate cortex in macaques. J Neurophysiol. 2005;94:3430–3442. - PubMed
-
- Bradley DC, Troyk PR, Berg JA, Bak M, Cogan S, Erickson R, Kufta C, Mascaro M, McCreery D, Schmidt EM, Towle VL, Xu H. Visuotopic mapping through a multichannel stimulating implant in primate V1. J Neurophysiol. 2005;93:1659–1670. - PubMed
-
- Budinger E, Heil P, Scheich H. Functional organization of auditory cortex in the Mongolian gerbil (Meriones unguiculatus). III. Anatomical subdivisions and corticocortical connections. Eur J Neurosci. 2000;12:2425–2451. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
