Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus
- PMID: 20016107
- PMCID: PMC2824179
- DOI: 10.1523/JNEUROSCI.4041-09.2009
Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus
Abstract
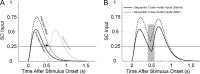
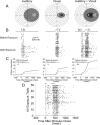
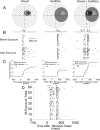
Multisensory neurons in the superior colliculus (SC) have the capability to integrate signals that belong to the same event, despite being conveyed by different senses. They develop this capability during early life as experience is gained with the statistics of cross-modal events. These adaptations prepare the SC to deal with the cross-modal events that are likely to be encountered throughout life. Here, we found that neurons in the adult SC can also adapt to experience with sequentially ordered cross-modal (visual-auditory or auditory-visual) cues, and that they do so over short periods of time (minutes), as if adapting to a particular stimulus configuration. This short-term plasticity was evident as a rapid increase in the magnitude and duration of responses to the first stimulus, and a shortening of the latency and increase in magnitude of the responses to the second stimulus when they are presented in sequence. The result was that the two responses appeared to merge. These changes were stable in the absence of experience with competing stimulus configurations, outlasted the exposure period, and could not be induced by equivalent experience with sequential within-modal (visual-visual or auditory-auditory) stimuli. A parsimonious interpretation is that the additional SC activity provided by the second stimulus became associated with, and increased the potency of, the afferents responding to the preceding stimulus. This interpretation is consistent with the principle of spike-timing-dependent plasticity, which may provide the basic mechanism for short term or long term plasticity and be operative in both the adult and neonatal SC.
Figures









References
-
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol. 2007b;97:3193–3205. - PubMed
-
- Bell AH, Meredith MA, Van Opstal AJ, Munoz DP. Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp Brain Res. 2006;174:53–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
