Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium
- PMID: 20016202
- PMCID: PMC2895757
- DOI: 10.1159/000265562
Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium
Abstract
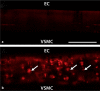
Background/aims: Myoendothelial junctions (MEJs) represent a specialized signaling domain between vascular smooth muscle cells (VSMC) and endothelial cells (EC). The functional consequences of phosphorylation state of the connexins (Cx) at the MEJ have not been explored.
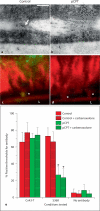
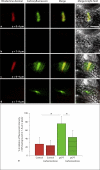
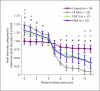
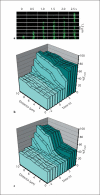
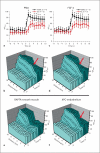
Methods/results: Application of adenosine 3',5'-cyclic monophosphate sodium (pCPT) to mouse cremasteric arterioles reduces the detection of connexin 43 (Cx43) phosphorylated at its carboxyl terminal serine 368 site (S368) at the MEJ in vivo. After single-cell microinjection of a VSMC in mouse cremaster arterioles, only in the presence of pCPT was dye transfer to EC observed. We used a vascular cell co-culture (VCCC) and applied the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (PMA) or fibroblast growth factor-2 (FGF-2) to induce phosphorylation of Cx43 S368. This phosphorylation event was associated with a significant reduction in dye transfer and calcium communication. Using a novel method to monitor increases in intracellular calcium across the in vitro MEJ, we noted that PMA and FGF-2 both inhibited movement of inositol 1,4,5-triphosphate (IP(3)), but to a lesser extent Ca(2+).
Conclusion: These data indicate that site-specific connexin phosphorylation at the MEJ can potentially regulate the movement of solutes between EC and VSMC in the vessel wall.
Copyright 2009 S. Karger AG, Basel.
Figures
References
-
- de Wit C, Boettcher M, Schmidt VJ. Signaling across myoendothelial gap junctions –fact or fiction? Cell Commun Adhes. 2008;15:231–245. - PubMed
-
- Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2009;36:67–76. - PubMed
-
- Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. - PubMed
-
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

