Stainless steel ions stimulate increased thrombospondin-1-dependent TGF-beta activation by vascular smooth muscle cells: implications for in-stent restenosis
- PMID: 20016205
- PMCID: PMC2895758
- DOI: 10.1159/000265565
Stainless steel ions stimulate increased thrombospondin-1-dependent TGF-beta activation by vascular smooth muscle cells: implications for in-stent restenosis
Abstract
Background/aims: Despite advances in stent design, in-stent restenosis (ISR) remains a significant clinical problem. All implant metals exhibit corrosion, which results in release of metal ions. Stainless steel (SS), a metal alloy widely used in stents, releases ions to the vessel wall and induces reactive oxygen species, inflammation and fibroproliferative responses. The molecular mechanisms are unknown. TGF-beta is known to be involved in the fibroproliferative responses of vascular smooth muscle cells (VSMCs) in restenosis, and TGF-beta antagonists attenuate ISR. We hypothesized that SS ions induce the latent TGF-beta activator, thrombospondin-1 (TSP1), through altered oxidative signaling to stimulate increased TGF-beta activation and VSMC phenotype change.
Methods: VSMCs were treated with SS metal ion cocktails, and morphology, TSP1, extracellular matrix production, desmin and TGF-beta activity were assessed by immunoblotting.
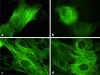
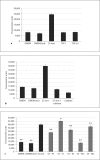
Results: SS ions stimulate the synthetic phenotype, increased TGF-beta activity, TSP1, increased extracellular matrix and downregulation of desmin in VSMCs. Furthermore, SS ions increase hydrogen peroxide and decrease cGMP-dependent protein kinase (PKG) signaling, a known repressor of TSP1 transcription. Catalase blocks SS ion attenuation of PKG signaling and increased TSP1 expression.
Conclusions: These data suggest that ions from stent alloy corrosion contribute to ISR through stimulation of TSP1-dependent TGF-beta activation.
Copyright 2009 S. Karger AG, Basel.
Figures







Similar articles
-
Glucose downregulation of PKG-I protein mediates increased thrombospondin1-dependent TGF-{beta} activity in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2010 May;298(5):C1188-97. doi: 10.1152/ajpcell.00330.2009. Epub 2010 Feb 17. Am J Physiol Cell Physiol. 2010. PMID: 20164378 Free PMC article.
-
Two-dimensional fluorescence in-gel electrophoresis of coronary restenosis tissues in minipigs: increased adipocyte fatty acid binding protein induces reactive oxygen species-mediated growth and migration in smooth muscle cells.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):572-80. doi: 10.1161/ATVBAHA.112.301016. Epub 2013 Jan 31. Arterioscler Thromb Vasc Biol. 2013. PMID: 23372061
-
Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells.J Biol Chem. 2002 Mar 22;277(12):9880-8. doi: 10.1074/jbc.M108360200. Epub 2002 Jan 9. J Biol Chem. 2002. PMID: 11784717
-
Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy.Circ J. 2011;75(6):1287-96. doi: 10.1253/circj.cj-11-0366. Epub 2011 Apr 29. Circ J. 2011. PMID: 21532177 Review.
-
Transforming growth factor-beta: a promising target for anti-stenosis therapy.Cardiovasc Drug Rev. 2001 Winter;19(4):329-44. doi: 10.1111/j.1527-3466.2001.tb00074.x. Cardiovasc Drug Rev. 2001. PMID: 11830751 Review.
Cited by
-
Enhanced biocompatibility of CD47-functionalized vascular stents.Biomaterials. 2016 May;87:82-92. doi: 10.1016/j.biomaterials.2016.02.008. Epub 2016 Feb 8. Biomaterials. 2016. PMID: 26914699 Free PMC article.
-
A neutralizing IL-11 antibody reduces vessel hyperplasia in a mouse carotid artery wire injury model.Sci Rep. 2021 Oct 19;11(1):20674. doi: 10.1038/s41598-021-99880-y. Sci Rep. 2021. PMID: 34667238 Free PMC article.
-
The complex regulation of TGF-β in cardiovascular disease.Vasc Health Risk Manag. 2012;8:533-9. doi: 10.2147/VHRM.S28041. Epub 2012 Sep 13. Vasc Health Risk Manag. 2012. PMID: 23028232 Free PMC article. Review.
-
Substrate-induced phenotypic switches of human smooth muscle cells: an in vitro study of in-stent restenosis activation pathways.J R Soc Interface. 2011 May 6;8(58):641-9. doi: 10.1098/rsif.2010.0532. Epub 2010 Nov 24. J R Soc Interface. 2011. PMID: 21106574 Free PMC article.
-
Stent thrombosis: understanding and managing a critical problem.Curr Treat Options Cardiovasc Med. 2012 Feb;14(1):91-107. doi: 10.1007/s11936-011-0155-4. Curr Treat Options Cardiovasc Med. 2012. PMID: 22193981
References
-
- Scott NA. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv Drug Deliv Rev. 2006;58:358–376. - PubMed
-
- Santin M, Colombo P, Bruschi G. Interfacial biology of in-stent restenosis. Expert Rev Med Devices. 2005;2:429–443. - PubMed
-
- Celik T, Iyisoy A, Jata B, Yuksel CU, Isik E. Stent fracture: a new villain of the village. Int J Cardiol. 2008 E-pub ahead of print. - PubMed
-
- Kim JS, Lee SY, Lee JM, Yoon YW, Ahn CM, Kim MH, Min PK, Ko YG, Hong BK, Choi D, Kwon HM, Jang Y, Shim WH. Significant association of coronary stent fracture with in-stent restenosis in sirolimus-eluting stents. Coron Artery Dis. 2009;20:59–63. - PubMed
-
- Rits J, van Herwaarden JA, Jahrome AK, Krievins D, Moll FL. The incidence of arterial stent fractures with exclusion of coronary, aortic, and non-arterial settings. Eur J Vasc Endovasc Surg. 2008;36:339–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EB001715/EB/NIBIB NIH HHS/United States
- F30 DE018259/DE/NIDCR NIH HHS/United States
- HL044195/HL/NHLBI NIH HHS/United States
- R01 HL080017/HL/NHLBI NIH HHS/United States
- HL080017/HL/NHLBI NIH HHS/United States
- F30DE018259/DE/NIDCR NIH HHS/United States
- C06 RR 15490/RR/NCRR NIH HHS/United States
- C06 RR015490/RR/NCRR NIH HHS/United States
- R01 EB001715/EB/NIBIB NIH HHS/United States
- HL079644/HL/NHLBI NIH HHS/United States
- R01 HL079644/HL/NHLBI NIH HHS/United States
- DK078038/DK/NIDDK NIH HHS/United States
- R01 DK060658/DK/NIDDK NIH HHS/United States
- R01 DK078038/DK/NIDDK NIH HHS/United States
- R01 HL044195/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

