TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells
- PMID: 20016274
- PMCID: PMC3664275
- DOI: 10.4161/cc.9.1.10358
TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells
Abstract
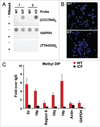
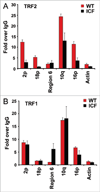
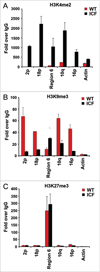
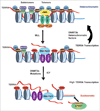
Self-reinforcing negative feedback loops are commonly observed in biological systems. RNA-mediated negative feedback loops have been described in the formation of heterochromatin at centromeres in fission yeast and the inactive X chromosome in mammalian cells. The telomere repeat-containing RNA (TERRA) has also been implicated in the formation of telomeric heterochromatin through a self-reinforcing negative feedback loop. In cells derived from human ICF syndrome, TERRA levels are abnormally elevated and telomeres are abnormally shortened. We now show that telomere heterochromatin is also abnormal in ICF cells. We propose that ICF cells fail to reinforce the TERRA-dependent negative feedback loop as a result of the inability to establish heterochromatin at subtelomeres. This failure is likely due to the lack of DNMT3b and DNA methylation, which is a genetic lesion associated with ICF syndrome. Failure of this feedback mechanism leads to catastrophic telomere dysfunction and chromosome instability.
Figures
Similar articles
-
Telomeric noncoding RNA: telomeric repeat-containing RNA in telomere biology.Wiley Interdiscip Rev RNA. 2014 May-Jun;5(3):407-19. doi: 10.1002/wrna.1220. Epub 2014 Feb 12. Wiley Interdiscip Rev RNA. 2014. PMID: 24523222 Review.
-
Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions.Hum Mol Genet. 2008 Sep 15;17(18):2776-89. doi: 10.1093/hmg/ddn177. Epub 2008 Jun 16. Hum Mol Genet. 2008. PMID: 18558631
-
Subtelomeric methylation distinguishes between subtypes of Immunodeficiency, Centromeric instability and Facial anomalies syndrome.Hum Mol Genet. 2018 Oct 15;27(20):3568-3581. doi: 10.1093/hmg/ddy265. Hum Mol Genet. 2018. PMID: 30010917
-
Induced pluripotent stem cells as a model for telomeric abnormalities in ICF type I syndrome.Hum Mol Genet. 2014 Jul 15;23(14):3629-40. doi: 10.1093/hmg/ddu071. Epub 2014 Feb 18. Hum Mol Genet. 2014. PMID: 24549038
-
TERRA: telomeric repeat-containing RNA.EMBO J. 2009 Sep 2;28(17):2503-10. doi: 10.1038/emboj.2009.166. Epub 2009 Jul 23. EMBO J. 2009. PMID: 19629047 Free PMC article. Review.
Cited by
-
Bring It to an End: Does Telomeres Size Matter?Cells. 2019 Jan 8;8(1):30. doi: 10.3390/cells8010030. Cells. 2019. PMID: 30626097 Free PMC article. Review.
-
Noncoding RNAs Controlling Telomere Homeostasis in Senescence and Aging.Trends Mol Med. 2020 Apr;26(4):422-433. doi: 10.1016/j.molmed.2020.01.010. Epub 2020 Feb 28. Trends Mol Med. 2020. PMID: 32277935 Free PMC article. Review.
-
The relationship between DNA methylation and telomere length in dyskeratosis congenita.Aging Cell. 2012 Feb;11(1):24-8. doi: 10.1111/j.1474-9726.2011.00755.x. Epub 2011 Nov 15. Aging Cell. 2012. PMID: 21981348 Free PMC article.
-
Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity.Front Genet. 2015 Apr 14;6:143. doi: 10.3389/fgene.2015.00143. eCollection 2015. Front Genet. 2015. PMID: 25926849 Free PMC article. Review.
-
Non-coding telomeric and subtelomeric transcripts are differentially regulated by telomeric and heterochromatin assembly factors in fission yeast.Nucleic Acids Res. 2012 Apr;40(7):2956-63. doi: 10.1093/nar/gkr1155. Epub 2011 Dec 1. Nucleic Acids Res. 2012. PMID: 22139922 Free PMC article.
References
-
- Azzalin CM, Reichenback P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance ractors at mammalian chromosome ends. Science. 2007;318:798–801. - PubMed
-
- Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10:228–236. - PubMed
-
- Azzalin CM, Lingner J. Telomeres: the silence is broken. Cell Cycle. 2008;7:1161–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
