Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report
- PMID: 20016531
- PMCID: PMC2906139
- DOI: 10.1038/leu.2009.262
Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report
Abstract
The Children's Cancer Group enrolled 13 298 young people age <21 years on 1 of 16 protocols between 1983 and 2002. Outcomes were examined in three time periods, 1983-1988, 1989-1995, 1996-2002. Over the three intervals, 10-year event-free survival (EFS) for Rome/National Cancer Institute standard risk (SR) and higher risk (HR) B-precursor patients was 68 and 58%, 77 and 63%, and 78 and 67%, respectively, whereas for SR and HR T-cell patients, EFS was 65 and 56%, 78 and 68%, and 70 and 72%, respectively. Five-year EFS for infants was 36, 38, and 43%, respectively. Seminal randomized studies led to a number of important findings. Stronger post-induction intensification improved outcome for both SR and HR patients. With improved systemic therapy, additional intrathecal (IT) methotrexate effectively replaced cranial radiation. For SR patients receiving three-drug induction, iso-toxic substitution of dexamethasone for prednisone improved EFS. Pegylated asparaginase safely and effectively replaced native asparaginase. Thus, rational therapy modifications yielded better outcomes for both SR and HR patients. These trials provide the platforms for current Children's Oncology Group trials.
Figures
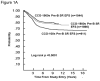
Standard risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; SR, Standard risk; EFS, event-free survival
Higher risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; HR, high risk; EFS, event free survival

Standard risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; SR, Standard risk; EFS, event-free survival
Higher risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; HR, high risk; EFS, event free survival
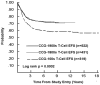

CCG-100 Series (1983–1988)
CCG-1800 Series (1989–1995)
CCG-1900 Series (1996–2002)

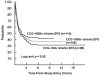
CCG-100 Series (1983–1988)
CCG-1800 Series (1989–1995)
CCG-1900 Series (1996–2002)

CCG-100 Series (1983–1988)
CCG-1800 Series (1989–1995)
CCG-1900 Series (1996–2002)
References
-
- Pinkel D, Simone J, Hustu HO, Aur RJ. Nine years’ experience with “total therapy” of childhood acute lymphocytic leukemia. Pediatr. 1972;50(2):246–251. - PubMed
-
- Henze G, Langermann HJ, Bramswig J, Breu H, Gadner H, Schellong G, Welte K, Riehm H. The BFM 76/79 acute lymphoblastic leukemia therapy study (author’s transl) Klin Padiatr. 1981;193(3):145–154. - PubMed
-
- Bleyer WA, Coccia PF, Sather HN, Level C, Lukens J, Niebrugge DJ, Siegel S, Littman PS, Leikin SL, Miller DR, et al. Reduction in central nervous system leukemia with a pharmacokinetically derived IT methotrexate dosage regimen. J Clin Oncol. 1983;1(5):317–325. - PubMed
-
- Miller DR, Leikin S, Albo V, Sather H, Karon M, Hammond D. Prognostic factors and therapy in acute lymphoblastic leukemia of childhood: CCG-141. A report from the Children’s Cancer Study Group. Cancer. 1983;51(6):1041–1049. - PubMed
-
- Bleyer WA, Sather HN, Nickerson HJ, Coccia PF, Finklestein JZ, Miller DR, et al. Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low-risk childhood acute lymphoblastic leukemia: a report of the CCG-161 study by the Childrens Cancer Study Group. J Clin Oncol. 1991;9(6):1012–1021. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

