Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000)
- PMID: 20016537
- PMCID: PMC2820141
- DOI: 10.1038/leu.2009.253
Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000)
Abstract
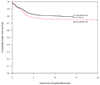
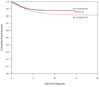
The Dana-Farber Cancer Institute (DFCI) acute lymphoblastic leukemia (ALL) Consortium has been conducting multi-institutional clinical trials in childhood ALL since 1981. The treatment backbone has included 20-30 consecutive weeks of asparaginase during intensification and frequent vincristine/corticosteroid pulses during the continuation phase. Between 1985 and 2000, 1457 children aged 0-18 years were treated on four consecutive protocols: 85-01 (1985-1987), 87-01 (1987-1991), 91-01 (1991-1955) and 95-01 (1996-2000). The 10-year event-free survival (EFS)+/-s.e. by protocol was 77.9+/-2.8% (85-01), 74.2+/-2.3% (87-01), 80.8+/-2.1% (91-01) and 80.5+/-1.8% (95-01). Approximately 82% of patients treated in the 1980s and 88% treated in the 1990s were long-term survivors. Both EFS and overall survival (OS) rates were significantly higher for patients treated in the 1990s compared with the 1980s (P=0.05 and 0.01, respectively). On the two protocols conducted in the 1990s, EFS was 79-85% for T-cell ALL patients and 75-78% for adolescents (age 10-18 years). Results of randomized studies revealed that dexrazoxane prevented acute cardiac injury without adversely affecting EFS or OS in high-risk (HR) patients, and frequently dosed intrathecal chemotherapy was an effective substitute for cranial radiation in standard-risk (SR) patients. Current studies continue to focus on improving efficacy while minimizing acute and late toxicities.
Conflict of interest statement
Figures




References
-
- Sallan SE, Cammita BM, Cassady JR, Nathan DG, Frei E., 3rd Intermittent combination chemotherapy with adriamycin for childhood acute lymphoblastic leukemia: clinical results. Blood. 1978;51(3):425–433. - PubMed
-
- Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E, 3rd, Nathan DG. Influence of intensive asparaginase in the treatment of childhood non-T- cell acute lymphoblastic leukemia. Cancer Res. 1983;43(11):5601–5607. - PubMed
-
- Clavell LA, Gelber RD, Cohen HJ, Hitchcock-Bryan S, Cassady JR, Tarbell NJ, et al. Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med. 1986;315(11):657–663. - PubMed
-
- Schorin MA, Blattner S, Gelber RD, Tarbell NJ, Donnelly M, Dalton V, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana- Farber Cancer Institute/Children's Hospital Acute Lymphoblastic Leukemia Consortium Protocol 85-01. J Clin Oncol. 1994;12(4):740–747. - PubMed
-
- LeClerc JM, Billett AL, Gelber RD, Dalton V, Tarbell N, Lipton JM, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana- Farber ALL Consortium Protocol 87-01. J Clin Oncol. 2002;20(1):237–246. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

