Relative dose intensity delivered to patients with early breast cancer: Canadian experience
- PMID: 20016741
- PMCID: PMC2794674
- DOI: 10.3747/co.v16i6.311
Relative dose intensity delivered to patients with early breast cancer: Canadian experience
Abstract
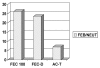
Adjuvant chemotherapy for early breast cancer improves disease-free and overall survival in pre- and postmenopausal women. The importance of maintaining relative dose intensity (RDI) is well-known; however, little information is available from routine clinical practice regarding how well dose intensity is maintained with modern chemotherapy regimens.In a retrospective review of patients undergoing chemotherapy for early breast cancer at a single institution in Canada from January 2006 to November 2007, a total of 263 patients received one of the following regimens:AC-T [doxorubicin (Adriamycin: Pharmacia, Kalamazoo, MI, U.S.A.)-cyclophosphamide, paclitaxel (Taxol: Bristol-Myers Squibb, Princeton, NJ, U.S.A.)]FEC-100 (5-fluorouracil-epirubicin-cyclophosphamide)FEC-D (5-fluorouracil-epirubicin-cyclophosphamide, docetaxel)Overall, only 14.4% of patients had a RDI less than 85%. Dose delay or reduction (or both) occurred in 46%, 37%, and 20% of patients receiving fec-100, ac-t, and fec-d respectively. Optimal RDI was delivered to 96%, 95%, and 70.7% of patients for ac-t, fec-d and fec-100 regimens respectively. Patients over 65 years of age accounted for 14% of the total cohort and were more likely to receive a suboptimal RDI than were patients younger than 65 years of age (35% vs. 6.6%).Optimal chemotherapy RDI (>85%) for early breast cancer can be achieved at an academic cancer centre. This goal is less often accomplished in elderly patients, and thus a proactive approach is required for managing toxicity in that population.
Keywords: Early breast cancer; adjuvant chemotherapy; relative dose intensity.
Figures


Similar articles
-
Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial.Lancet. 2015 May 9;385(9980):1863-72. doi: 10.1016/S0140-6736(14)62048-1. Epub 2015 Mar 2. Lancet. 2015. PMID: 25740286 Clinical Trial.
-
Maintaining Dose Intensity of Adjuvant Chemotherapy in Older Patients With Breast Cancer.Clin Breast Cancer. 2018 Oct;18(5):e1181-e1187. doi: 10.1016/j.clbc.2018.04.016. Epub 2018 Apr 28. Clin Breast Cancer. 2018. PMID: 29778788
-
Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice.Eur J Cancer. 2017 Jul;79:152-157. doi: 10.1016/j.ejca.2017.04.001. Epub 2017 May 8. Eur J Cancer. 2017. PMID: 28494406
-
Optimizing adjuvant breast cancer chemotherapy: rationale for the MA.21 study.Oncology (Williston Park). 2001 May;15(5 Suppl 7):7-13. Oncology (Williston Park). 2001. PMID: 11396366 Review.
-
Epirubicin: a review of its efficacy as adjuvant therapy and in the treatment of metastatic disease in breast cancer.Drugs Aging. 1999 Nov;15(5):389-416. doi: 10.2165/00002512-199915050-00006. Drugs Aging. 1999. PMID: 10600046 Review.
Cited by
-
CYP2J2∗7 Genotype Predicts Risk of Chemotherapy-Induced Hematologic Toxicity and Reduced Relative Dose Intensity in Ethiopian Breast Cancer Patients.Front Pharmacol. 2019 May 14;10:481. doi: 10.3389/fphar.2019.00481. eCollection 2019. Front Pharmacol. 2019. PMID: 31139078 Free PMC article.
-
Dose Delays, Dose Reductions, and Relative Total Dose Intensity in Patients With Advanced Cancer Who Exercised During Neoadjuvant Chemotherapy Treatment.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231168368. doi: 10.1177/15347354231168368. Integr Cancer Ther. 2023. PMID: 37077136 Free PMC article.
-
Proof of Concept of a Mobile Health Short Message Service Text Message Intervention That Promotes Adherence to Oral Anticancer Agent Medications: A Randomized Controlled Trial.Telemed J E Health. 2016 Jun;22(6):497-506. doi: 10.1089/tmj.2015.0126. Epub 2015 Dec 30. Telemed J E Health. 2016. PMID: 26716365 Free PMC article. Clinical Trial.
-
Randomised controlled trial of intermittent vs continuous energy restriction during chemotherapy for early breast cancer.Br J Cancer. 2022 May;126(8):1157-1167. doi: 10.1038/s41416-021-01650-0. Epub 2021 Dec 15. Br J Cancer. 2022. PMID: 34912072 Free PMC article. Clinical Trial.
-
Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis.Support Care Cancer. 2012 Oct;20(10):2523-30. doi: 10.1007/s00520-011-1375-6. Epub 2012 Jan 15. Support Care Cancer. 2012. PMID: 22252548
References
-
- Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2006. Toronto: Canadian Cancer Society; 2006.
-
- Early Breast Cancer Trialists’ Collaborative Group (ebctcg) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717. - PubMed
-
- Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–6. - PubMed
-
- Kwak LW, Halpern J, Olshen RA, Horning SJ. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–77. - PubMed
-
- Lepage E, Gisselbrecht C, Haioun C, et al. Prognostic significance of received relative dose intensity in non-Hodgkin’s lymphoma patients: application to lnh-87 protocol. The gela (Groupe d’Etude des Lymphomes de l’Adulte) Ann Oncol. 1993;4:651–6. - PubMed
LinkOut - more resources
Full Text Sources

