Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer
- PMID: 20016752
- PMCID: PMC2793426
- DOI: 10.1155/2010/617421
Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer
Abstract
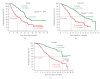
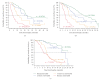
The increasing number of treatment options for patients with metastatic carcinomas has created a concomitant need for new methods to monitor their use. Ideally, these modalities would be noninvasive, be independent of treatment, and provide quantitative real-time analysis of tumor activity in a variety of carcinomas. Assessment of circulating tumor cells (CTCs) shed into the blood during metastasis may satisfy this need. We developed the CellSearch System to enumerate CTC from 7.5 mL of venous blood. In this review we compare the outcomes from three prospective multicenter studies investigating the use of CTC to monitor patients undergoing treatment for metastatic breast (MBC), colorectal (MCRC), or prostate cancer (MPC) and review the CTC definition used in these studies. Evaluation of CTC at anytime during the course of disease allows assessment of patient prognosis and is predictive of overall survival.
Figures


References
-
- Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. The Medical Journal of Australia. 1869;14:146–147.
-
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer. 2003;3(6):453–458. - PubMed
-
- Sleijfer S, Gratama J-W, Sieuwerts AM, Kraan J, Martens JWM, Foekens JA. Circulating tumour cell detection on its way to routine diagnostic implementation? European Journal of Cancer. 2007;43(18):2645–2650. - PubMed
-
- Hayes DF, Smerage JR. Is there a role for circulating tumor cells in the management of breast cancer? Clinical Cancer Research. 2008;14(12):3646–3650. - PubMed
-
- Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nature Reviews Clinical Oncology. 2009;6(6):339–351. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

