Novel Nucleoside Analogues with Fluorophores Replacing the DNA Base
- PMID: 20016809
- PMCID: PMC2788824
- DOI: 10.1002/(sici)1522-2675(19991215)82:12<2160::aid-hlca2160>3.0.co;2-4
Novel Nucleoside Analogues with Fluorophores Replacing the DNA Base
Abstract
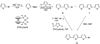
We describe the preparation and fluorescence properties of a set of new nucleosides in which a known hydrocarbon or oligothiophene fluorophore replaces the DNA base at C(1) of the deoxyribose moiety (see 3a - f). These compounds are potentially useful as probes in the study of the structure and dynamics of nucleic acids and their complexes with proteins. In addition, they may find use as fluorescent labels for nucleic-acid-based biomedical diagnostics methods. The fluorophores conjugated to deoxyribose at C(1) in the α-d-form include terphenyl, stilbene, terthiophene, benzoterthiophene, and pyrene. Also included is a non-fluorescent spacer in which cyclohexene replaces the DNA base. The nucleosides are derived from brominated fluorophore precursors and Hoffer's 2-deoxy-3,5-di-O-(p-toluoyl)-d-ribofuranosyl chloride. The emission maxima of the free nucleosides range from 345 to 536 nm. Also described are the 5'-(dimethoxytrityl) 3'-O-phosphoramidite derivatives 5a - f, suitable for incorporation into oligonucleotides by automated synthesizers.
Figures
Similar articles
-
Oligomeric fluorescent labels for DNA.Bioconjug Chem. 2005 May-Jun;16(3):528-34. doi: 10.1021/bc0497766. Bioconjug Chem. 2005. PMID: 15898718
-
Quenching of fluorescent nucleobases by neighboring DNA: the "insulator" concept.Chembiochem. 2008 Jan 25;9(2):279-85. doi: 10.1002/cbic.200700381. Chembiochem. 2008. PMID: 18072185
-
Naphthalene, Phenanthrene, and Pyrene as DNA Base Analogues: Synthesis, Structure, and Fluorescence in DNA.J Am Chem Soc. 1996 Aug 21;118(33):7671-7678. doi: 10.1021/ja9612763. J Am Chem Soc. 1996. PMID: 20865136 Free PMC article.
-
Environmentally sensitive fluorescent nucleoside analogues as probes for nucleic acid - protein interactions: molecular design and biosensing applications.Methods Appl Fluoresc. 2022 Jul 4;10(4). doi: 10.1088/2050-6120/ac7bd8. Methods Appl Fluoresc. 2022. PMID: 35738250 Review.
-
Analytical ancestry: "firsts" in fluorescent labeling of nucleosides, nucleotides, and nucleic acids.Clin Chem. 2009 Apr;55(4):670-83. doi: 10.1373/clinchem.2008.116152. Epub 2009 Feb 20. Clin Chem. 2009. PMID: 19233914 Review.
Cited by
-
Genetically encoded multispectral labeling of proteins with polyfluorophores on a DNA backbone.J Am Chem Soc. 2013 Apr 24;135(16):6184-91. doi: 10.1021/ja4004393. Epub 2013 Apr 16. J Am Chem Soc. 2013. PMID: 23590213 Free PMC article.
-
Polyfluorophores on a DNA backbone: a multicolor set of labels excited at one wavelength.J Am Chem Soc. 2009 Mar 25;131(11):3923-33. doi: 10.1021/ja805502k. J Am Chem Soc. 2009. PMID: 19254023 Free PMC article.
-
Fluorescent analogs of biomolecular building blocks: design, properties, and applications.Chem Rev. 2010 May 12;110(5):2579-619. doi: 10.1021/cr900301e. Chem Rev. 2010. PMID: 20205430 Free PMC article. Review. No abstract available.
-
Pattern-based detection of toxic metals in surface water with DNA polyfluorophores.Angew Chem Int Ed Engl. 2014 May 19;53(21):5361-5. doi: 10.1002/anie.201403235. Epub 2014 Apr 22. Angew Chem Int Ed Engl. 2014. PMID: 24756982 Free PMC article.
-
Fluorescent pyrimidine ribonucleotide: synthesis, enzymatic incorporation, and utilization.J Am Chem Soc. 2007 Feb 21;129(7):2044-53. doi: 10.1021/ja066455r. Epub 2007 Jan 26. J Am Chem Soc. 2007. PMID: 17256858 Free PMC article.
References
-
- Beaucage SL, Iyer RP. Tetrahedron. 1993;49:1925.
- Cunningham RE. Methods Mol. Biol. 1999;115:271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources