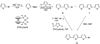
Novel Nucleoside Analogues with Fluorophores Replacing the DNA Base
- PMID: 20016809
- PMCID: PMC2788824
- DOI: 10.1002/(sici)1522-2675(19991215)82:12<2160::aid-hlca2160>3.0.co;2-4
Novel Nucleoside Analogues with Fluorophores Replacing the DNA Base
Abstract
We describe the preparation and fluorescence properties of a set of new nucleosides in which a known hydrocarbon or oligothiophene fluorophore replaces the DNA base at C(1) of the deoxyribose moiety (see 3a - f). These compounds are potentially useful as probes in the study of the structure and dynamics of nucleic acids and their complexes with proteins. In addition, they may find use as fluorescent labels for nucleic-acid-based biomedical diagnostics methods. The fluorophores conjugated to deoxyribose at C(1) in the α-d-form include terphenyl, stilbene, terthiophene, benzoterthiophene, and pyrene. Also included is a non-fluorescent spacer in which cyclohexene replaces the DNA base. The nucleosides are derived from brominated fluorophore precursors and Hoffer's 2-deoxy-3,5-di-O-(p-toluoyl)-d-ribofuranosyl chloride. The emission maxima of the free nucleosides range from 345 to 536 nm. Also described are the 5'-(dimethoxytrityl) 3'-O-phosphoramidite derivatives 5a - f, suitable for incorporation into oligonucleotides by automated synthesizers.
Figures
References
-
- Beaucage SL, Iyer RP. Tetrahedron. 1993;49:1925.
- Cunningham RE. Methods Mol. Biol. 1999;115:271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources