Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies
- PMID: 20016813
- PMCID: PMC2789379
- DOI: 10.1371/journal.pone.0008314
Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies
Abstract
Background: Recombinant monoclonal antibodies have emerged as important tools for cancer therapy. Despite the promise shown by antibody-based therapies, the large molecular size of antibodies limits their ability to efficiently penetrate solid tumors and precludes efficient crossing of the blood-brain-barrier into the central nervous system (CNS). Consequently, poorly vascularized solid tumors and CNS metastases cannot be effectively treated by intravenously-injected antibodies. The inherent tumor-tropic properties of human neural stem cells (NSCs) can potentially be harnessed to overcome these obstacles and significantly improve cancer immunotherapy. Intravenously-delivered NSCs preferentially migrate to primary and metastatic tumor sites within and outside the CNS. Therefore, we hypothesized that NSCs could serve as an ideal cellular delivery platform for targeting antibodies to malignant tumors.
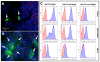
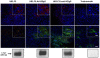
Methods and findings: As proof-of-concept, we selected Herceptin (trastuzumab), a monoclonal antibody widely used to treat HER2-overexpressing breast cancer. HER2 overexpression in breast cancer is highly correlated with CNS metastases, which are inaccessible to trastuzumab therapy. Therefore, NSC-mediated delivery of trastuzumab may improve its therapeutic efficacy. Here we report, for the first time, that human NSCs can be genetically modified to secrete anti-HER2 immunoglobulin molecules. These NSC-secreted antibodies assemble properly, possess tumor cell-binding affinity and specificity, and can effectively inhibit the proliferation of HER2-overexpressing breast cancer cells in vitro. We also demonstrate that immunoglobulin-secreting NSCs exhibit preferential tropism to tumor cells in vivo, and can deliver antibodies to human breast cancer xenografts in mice.
Conclusions: Taken together, these results suggest that NSCs modified to secrete HER2-targeting antibodies constitute a promising novel platform for targeted cancer immunotherapy. Specifically, this NSC-mediated antibody delivery system has the potential to significantly improve clinical outcome for patients with HER2-overexpressing breast cancer.
Conflict of interest statement
Figures



References
-
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. - PubMed
-
- Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. - PubMed
-
- Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

