The catalytic subunit of protein phosphatase 1 gamma regulates thrombin-induced murine platelet alpha(IIb)beta(3) function
- PMID: 20016849
- PMCID: PMC2788699
- DOI: 10.1371/journal.pone.0008304
The catalytic subunit of protein phosphatase 1 gamma regulates thrombin-induced murine platelet alpha(IIb)beta(3) function
Abstract
Background: Hemostasis and thrombosis are regulated by agonist-induced activation of platelet integrin alpha(IIb)beta(3). Integrin activation, in turn is mediated by cellular signaling via protein kinases and protein phosphatases. Although the catalytic subunit of protein phosphatase 1 (PP1c) interacts with alpha(IIb)beta(3), the role of PP1c in platelet reactivity is unclear.
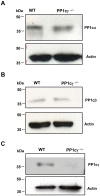
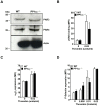
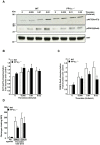
Methodology/principal findings: Using gamma isoform of PP1c deficient mice (PP1cgamma(-/-)), we show that the platelets have moderately decreased soluble fibrinogen binding and aggregation to low concentrations of thrombin or protease-activated receptor 4 (PAR4)-activating peptide but not to adenosine diphosphate (ADP), collagen or collagen-related peptide (CRP). Thrombin-stimulated PP1cgamma(-/-) platelets showed decreased alpha(IIb)beta(3) activation despite comparable levels of alpha(IIb)beta(3), PAR3, PAR4 expression and normal granule secretion. Functions regulated by outside-in integrin alpha(IIb)beta(3) signaling like adhesion to immobilized fibrinogen and clot retraction were not altered in PP1cgamma(-/-) platelets. Thrombus formation induced by a light/dye injury in the cremaster muscle venules was significantly delayed in PP1cgamma(-/-) mice. Phosphorylation of glycogen synthase kinase (GSK3)beta-serine 9 that promotes platelet function, was reduced in thrombin-stimulated PP1cgamma(-/-) platelets by an AKT independent mechanism. Inhibition of GSK3beta partially abolished the difference in fibrinogen binding between thrombin-stimulated wild type and PP1cgamma(-/-) platelets.
Conclusions/significance: These studies illustrate a role for PP1cgamma in maintaining GSK3beta-serine9 phosphorylation downstream of thrombin signaling and promoting thrombus formation via fibrinogen binding and platelet aggregation.
Conflict of interest statement
Figures



Similar articles
-
Dual regulation of glycogen synthase kinase 3 (GSK3)α/β by protein kinase C (PKC)α and Akt promotes thrombin-mediated integrin αIIbβ3 activation and granule secretion in platelets.J Biol Chem. 2013 Feb 8;288(6):3918-28. doi: 10.1074/jbc.M112.429936. Epub 2012 Dec 13. J Biol Chem. 2013. PMID: 23239877 Free PMC article.
-
Opposing Roles for the α Isoform of the Catalytic Subunit of Protein Phosphatase 1 in Inside-Out and Outside-In Integrin Signaling in Murine Platelets.Cells. 2023 Oct 10;12(20):2424. doi: 10.3390/cells12202424. Cells. 2023. PMID: 37887268 Free PMC article.
-
Protease-activated receptor-induced Akt activation--regulation and possible function.J Thromb Haemost. 2007 Dec;5(12):2484-93. doi: 10.1111/j.1538-7836.2007.02769.x. Epub 2007 Sep 19. J Thromb Haemost. 2007. PMID: 17883592
-
Protein kinase C-theta in platelet activation.FEBS Lett. 2011 Oct 20;585(20):3208-15. doi: 10.1016/j.febslet.2011.09.014. Epub 2011 Sep 17. FEBS Lett. 2011. PMID: 21944869 Review.
-
The integrin alpha IIb/beta 3 in human platelet signal transduction.Biochem Pharmacol. 2000 Oct 15;60(8):1069-74. doi: 10.1016/s0006-2952(00)00417-2. Biochem Pharmacol. 2000. PMID: 11007943 Review.
Cited by
-
Distinct roles for the α , β and γ1 isoforms of protein phosphatase 1 in the outside-in αIIbβ3 integrin signalling-dependent functions.Thromb Haemost. 2013 Jan;109(1):118-26. doi: 10.1160/TH12-04-0237. Epub 2012 Nov 29. Thromb Haemost. 2013. PMID: 23197154 Free PMC article.
-
Platelets and Complement Cross-Talk in Early Atherogenesis.Front Cardiovasc Med. 2019 Sep 6;6:131. doi: 10.3389/fcvm.2019.00131. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31555668 Free PMC article. Review.
-
Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting.J Hematol Oncol. 2019 Mar 7;12(1):26. doi: 10.1186/s13045-019-0709-6. J Hematol Oncol. 2019. PMID: 30845955 Free PMC article. Review.
-
The β isoform of the catalytic subunit of protein phosphatase 2B restrains platelet function by suppressing outside-in αII b β3 integrin signaling.J Thromb Haemost. 2014 Dec;12(12):2089-101. doi: 10.1111/jth.12761. Epub 2014 Nov 15. J Thromb Haemost. 2014. PMID: 25330904 Free PMC article.
-
New approaches in tail-bleeding assay in mice: improving an important method for designing new anti-thrombotic agents.Int J Exp Pathol. 2016 Jun;97(3):285-92. doi: 10.1111/iep.12182. Epub 2016 Jul 5. Int J Exp Pathol. 2016. PMID: 27377432 Free PMC article.
References
-
- Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. - PubMed
-
- Li Z, Xi X, Du X. Mitogen-activated Protein Kinase-dependent Signaling Pathway in the Activation of Platelet Integrin αIIbβ3. J Biol Chem. 2001;276:42226–42232. - PubMed
-
- Li Z, Zhang G, Marjanovic JA, Ruan C, Du X. A platelet secretion pathway mediated by cGMP-dependent protein kinase. J Biol Chem. 2004;279:42469–42475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

