Cooperation of tumor-derived HBx mutants and p53-249(ser) mutant in regulating cell proliferation, anchorage-independent growth and aneuploidy in a telomerase-immortalized normal human hepatocyte-derived cell line
- PMID: 20017137
- PMCID: PMC2950321
- DOI: 10.1002/ijc.25118
Cooperation of tumor-derived HBx mutants and p53-249(ser) mutant in regulating cell proliferation, anchorage-independent growth and aneuploidy in a telomerase-immortalized normal human hepatocyte-derived cell line
Abstract
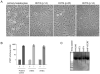
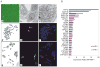
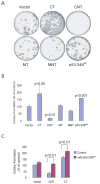
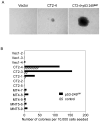
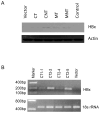
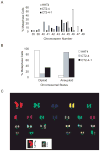
Hepatocellular carcinoma (HCC) is a common cancer, and hepatitis B virus (HBV) is a major etiological agent. Convincing epidemiological and experimental evidence also links HCC to aflatoxin, a naturally occurring mycotoxin that produces a signature p53-249(ser) mutation. Recently, we have reported that tumor-derived HBx variants encoded by HBV exhibited attenuated transactivation and proapoptotic functions but retained their ability to block p53-mediated apoptosis. These results indicate that mutations in HBx may contribute to the development of HCC. In this study, we determined whether tumor-derived HBx mutants along, or in cooperation with p53-249(ser), could alter cell proliferation and chromosome stability of normal human hepatocytes. To test this hypothesis, we established a telomerase immortalized normal human hepatocycte line HHT4 that exhibited a near diploid karyotype and expressed many hepatocyte-specific genes. We found that overexpression one of the tumor-derived HBx mutants, CT, significantly increased colony forming efficiency (CFE) while its corresponding wild-type allele CNT significantly decreased CFE in HHT4 cells. p53-249(ser) rescued CNT-mediated inhibition of colony formation. Although HHT4 cells lacked an anchorage independent growth capability as they did not form any colonies in soft agar, the CT-expressing HHT4 cells could form colonies, which could be significantly enhanced by p53-249(ser). Induction of aneuploidy could be observed in HHT4 cells expressing CT, but additionally recurring chromosome abnormalities could only be detected in cells coexpressing CT and p53-249(ser). Our results are consistent with the hypothesis that certain mutations in HBx and p53 at codon 249 may cooperate in contributing to liver carcinogenesis.
Figures
Similar articles
-
Hepatitis B virus X mutants derived from human hepatocellular carcinoma retain the ability to abrogate p53-induced apoptosis.Oncogene. 2001 Jun 21;20(28):3620-8. doi: 10.1038/sj.onc.1204495. Oncogene. 2001. PMID: 11439325
-
Hepatitis B virus X protein promotes hepatocellular carcinoma transformation through interleukin-6 activation of microRNA-21 expression.Eur J Cancer. 2014 Oct;50(15):2560-9. doi: 10.1016/j.ejca.2014.07.008. Epub 2014 Jul 30. Eur J Cancer. 2014. PMID: 25087183
-
Analysis of the tumorigenicity of the X gene of hepatitis B virus in a nontransformed hepatocyte cell line and the effects of cotransfection with a murine p53 mutant equivalent to human codon 249.Hepatology. 1996 Nov;24(5):1024-33. doi: 10.1002/hep.510240508. Hepatology. 1996. PMID: 8903370
-
Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma.J Gastroenterol Hepatol. 2011 Jan;26 Suppl 1:144-52. doi: 10.1111/j.1440-1746.2010.06546.x. J Gastroenterol Hepatol. 2011. PMID: 21199526 Review.
-
Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma.World J Gastroenterol. 2014 Aug 14;20(30):10238-48. doi: 10.3748/wjg.v20.i30.10238. World J Gastroenterol. 2014. PMID: 25132741 Free PMC article. Review.
Cited by
-
TP53 R249S mutation, genetic variations in HBX and risk of hepatocellular carcinoma in The Gambia.Carcinogenesis. 2012 Jun;33(6):1219-24. doi: 10.1093/carcin/bgs068. Carcinogenesis. 2012. PMID: 22759751 Free PMC article.
-
The effect of hydroxycamptothecin and pingyangmycin on human squamous cell carcinoma of the tongue.Oncol Lett. 2013 Mar;5(3):947-952. doi: 10.3892/ol.2013.1109. Epub 2013 Jan 7. Oncol Lett. 2013. PMID: 23426884 Free PMC article.
-
Global geographical overlap of aflatoxin and hepatitis C: controlling risk factors for liver cancer worldwide.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(3):534-40. doi: 10.1080/19440049.2012.751630. Epub 2013 Jan 2. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013. PMID: 23281740 Free PMC article.
-
RNA Sequencing of Hepatobiliary Cancer Cell Lines: Data and Applications to Mutational and Transcriptomic Profiling.Cancers (Basel). 2020 Sep 3;12(9):2510. doi: 10.3390/cancers12092510. Cancers (Basel). 2020. PMID: 32899426 Free PMC article.
-
Association of Aflatoxin and Gallbladder Cancer.Gastroenterology. 2017 Aug;153(2):488-494.e1. doi: 10.1053/j.gastro.2017.04.005. Epub 2017 Apr 17. Gastroenterology. 2017. PMID: 28428144 Free PMC article.
References
-
- Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12(9–10):S294–308. - PubMed
-
- Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–8. - PubMed
-
- Viviani S, Carrieri P, Bah E, Hall AJ, Kirk GD, Mendy M, Montesano R, Plymoth A, Sam O, Vander SM, Whittle H, Hainaut P. 20 years into the Gambia Hepatitis Intervention Study: assessment of initial hypotheses and prospects for evaluation of protective effectiveness against liver cancer. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3216–23. - PubMed
-
- Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007 Apr 2;26(15):2166–76. - PubMed
-
- Budhu A, Wang XW. The functional relevance of HBx subcellular localization and nuclear shuttling. In: Kobarg J, editor. The Pleiotropic Functions of the Viral Protein HBx in Hepatitis B Virus Infection and the Development of Liver Cancer. Kerala: Research Signpost; 2008. pp. 105–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

