Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish
- PMID: 20017210
- PMCID: PMC2841826
- DOI: 10.1002/cne.22214
Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish
Abstract
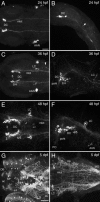
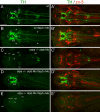
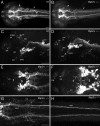
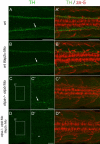
The catecholamines dopamine and noradrenaline provide some of the major neuromodulatory systems with far-ranging projections in the brain and spinal cord of vertebrates. However, development of these complex systems is only partially understood. Zebrafish provide an excellent model for genetic analysis of neuronal specification and axonal projections in vertebrates. Here, we analyze the ontogeny of the catecholaminergic projections in zebrafish embryos and larvae up to the fifth day of development and establish the basic scaffold of catecholaminergic connectivity. The earliest dopaminergic diencephalospinal projections do not navigate along the zebrafish primary neuron axonal scaffold but establish their own tracts at defined ventrolateral positions. By using genetic tools, we study quantitative and qualitative contributions of noradrenergic and defined dopaminergic groups to the catecholaminergic scaffold. Suppression of Tfap2a activity allows us to eliminate noradrenergic contributions, and depletion of Otp activity deletes mammalian A11-like Otp-dependent ventral diencephalic dopaminergic groups. This analysis reveals a predominant contribution of Otp-dependent dopaminergic neurons to diencephalospinal as well as hypothalamic catecholaminergic tracts. In contrast, noradrenergic projections make only a minor contribution to hindbrain and spinal catecholaminergic tracts. Furthermore, we can demonstrate that, in zebrafish larvae, ascending catecholaminergic projections to the telencephalon are generated exclusively by Otp-dependent diencephalic dopaminergic neurons as well as by hindbrain noradrenergic groups. Our data reveal the Otp-dependent A11-type dopaminergic neurons as the by far most prominent dopaminergic system in larval zebrafish. These findings are consistent with a hypothesis that Otp-dependent dopaminergic neurons establish the major modulatory system for somatomotor and somatosensory circuits in larval fish.
2009 Wiley-Liss, Inc.
Figures




Similar articles
-
Orthopedia transcription factor otpa and otpb paralogous genes function during dopaminergic and neuroendocrine cell specification in larval zebrafish.PLoS One. 2013 Sep 20;8(9):e75002. doi: 10.1371/journal.pone.0075002. eCollection 2013. PLoS One. 2013. PMID: 24073233 Free PMC article.
-
Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain.J Comp Neurol. 2010 Feb 15;518(4):423-38. doi: 10.1002/cne.22213. J Comp Neurol. 2010. PMID: 20017209 Free PMC article.
-
Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development.Curr Biol. 2007 May 15;17(10):873-80. doi: 10.1016/j.cub.2007.04.003. Epub 2007 May 3. Curr Biol. 2007. PMID: 17481897
-
Development of the dopamine systems in zebrafish.Adv Exp Med Biol. 2009;651:1-14. doi: 10.1007/978-1-4419-0322-8_1. Adv Exp Med Biol. 2009. PMID: 19731546 Review.
-
Dopaminergic and noradrenergic circuit development in zebrafish.Dev Neurobiol. 2012 Mar;72(3):256-68. doi: 10.1002/dneu.20911. Dev Neurobiol. 2012. PMID: 21567980 Review.
Cited by
-
Transient knockdown of tyrosine hydroxylase during development has persistent effects on behaviour in adult zebrafish (Danio rerio).PLoS One. 2012;7(8):e42482. doi: 10.1371/journal.pone.0042482. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879998 Free PMC article.
-
Parkinson-Associated SNCA Enhancer Variants Revealed by Open Chromatin in Mouse Dopamine Neurons.Am J Hum Genet. 2018 Dec 6;103(6):874-892. doi: 10.1016/j.ajhg.2018.10.018. Epub 2018 Nov 29. Am J Hum Genet. 2018. PMID: 30503521 Free PMC article.
-
Pla2g6 Deficiency in Zebrafish Leads to Dopaminergic Cell Death, Axonal Degeneration, Increased β-Synuclein Expression, and Defects in Brain Functions and Pathways.Mol Neurobiol. 2018 Aug;55(8):6734-6754. doi: 10.1007/s12035-017-0846-2. Epub 2018 Jan 17. Mol Neurobiol. 2018. PMID: 29344929
-
Early-life challenge enhances cortisol regulation in zebrafish larvae.Biol Open. 2024 Dec 15;13(12):bio061684. doi: 10.1242/bio.061684. Epub 2024 Nov 28. Biol Open. 2024. PMID: 39607018 Free PMC article.
-
2024 International Academy of Toxicologic Pathology (IATP) Satellite Symposium: New Approach Methodologies (NAMs) for Neurotoxicity Assessment and Regulatory Perspectives.Toxicol Pathol. 2025 Jun;53(4):305-320. doi: 10.1177/01926233251335719. Epub 2025 May 15. Toxicol Pathol. 2025. PMID: 40370040 Free PMC article.
References
-
- Björklund A, Dunnett SB. Dopamine neuron systems in the braan update. Trends Neurosci. 2007;30:194–202. - PubMed
-
- Björklund A, Skagerberg G. Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res. 1979;177:170–175. - PubMed
-
- Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. - PubMed
-
- Candy J, Collet C. Two tyrosine hydroxylase genes in teleosts. Biochim Biophys Acta. 2005;1727:35–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

