Evaluation of substituted N,N'-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase
- PMID: 20017496
- PMCID: PMC2818804
- DOI: 10.1021/jm901577g
Evaluation of substituted N,N'-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase
Abstract
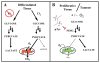
The metabolism of cancer cells is altered to support rapid proliferation. Pharmacological activators of a tumor cell specific pyruvate kinase isozyme (PKM2) may be an approach for altering the classic Warburg effect characteristic of aberrant metabolism in cancer cells yielding a novel antiproliferation strategy. In this manuscript, we detail the discovery of a series of substituted N,N'-diarylsulfonamides as activators of PKM2. The synthesis of numerous analogues and the evaluation of structure-activity relationships are presented as well as assessments of mechanism and selectivity. Several agents are found that have good potencies and appropriate solubility for use as chemical probes of PKM2 including 55 (AC(50) = 43 nM, maximum response = 84%; solubility = 7.3 microg/mL), 56 (AC(50) = 99 nM, maximum response = 84%; solubility = 5.7 microg/mL), and 58 (AC(50) = 38 nM, maximum response = 82%; solubility = 51.2 microg/mL). The small molecules described here represent first-in-class activators of PKM2.
Figures

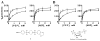
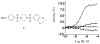
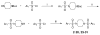
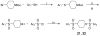
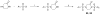
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
-
- Devita VT Jr, Hellman S, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. 7. Lippincott Williams & Wilkins; Philadelphia (PA): 2005.
-
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

