Regioselective intramolecular dipolar cycloaddition of azides and unsymmetrical alkynes
- PMID: 20017521
- PMCID: PMC3164318
- DOI: 10.1021/ol902681t
Regioselective intramolecular dipolar cycloaddition of azides and unsymmetrical alkynes
Abstract
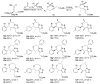
Enantioenriched allenylsilanes are used in three-component propargylation reactions with aldehydes and silyl ethers to form syn-homopropargylic ethers that contain an imbedded azide. These materials then undergo thermally induced intramolecular 1,3-dipolar cycloaddition reactions, resulting in unique fused ring systems containing 1,2,3-triazoles. The ability to modify all three components of the reaction allows for expedient access to compounds containing significant structural and stereochemical variation.
Figures
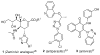
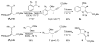


Similar articles
-
Synthesis of azide-alkyne fragments for "click" chemical applications. Part 2. Formation of oligomers from orthogonally protected chiral trialkylsilylhomopropargyl azides and homopropargyl alcohols.J Org Chem. 2010 Jan 15;75(2):390-8. doi: 10.1021/jo9021887. J Org Chem. 2010. PMID: 20000729
-
Rapid access to novel 1,2,3-triazolo-heterocyclic scaffolds via tandem Knoevenagel condensation/azide-alkyne 1,3-dipolar cycloaddition reaction in one pot.ACS Comb Sci. 2014 Sep 8;16(9):466-77. doi: 10.1021/co500070e. Epub 2014 Jun 26. ACS Comb Sci. 2014. PMID: 24945583
-
Facile entry into triazole fused heterocycles via sulfamidate derived azido-alkynes.J Org Chem. 2009 Oct 2;74(19):7588-91. doi: 10.1021/jo9016748. J Org Chem. 2009. PMID: 19725506
-
Recent applications of the Cu(I)-catalysed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in carbohydrate chemistry.Org Biomol Chem. 2007 Apr 7;5(7):1006-17. doi: 10.1039/b618048p. Epub 2007 Mar 5. Org Biomol Chem. 2007. PMID: 17377651 Review.
-
Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes.Med Res Rev. 2008 Mar;28(2):278-308. doi: 10.1002/med.20107. Med Res Rev. 2008. PMID: 17763363 Review.
Cited by
-
Click Chemistry for Biofunctional Polymers: From Observing to Steering Cell Behavior.Chem Rev. 2024 Dec 11;124(23):13216-13300. doi: 10.1021/acs.chemrev.4c00251. Epub 2024 Dec 2. Chem Rev. 2024. PMID: 39621547 Free PMC article. Review.
-
A modular reaction pairing approach to the diversity-oriented synthesis of fused- and bridged-polycyclic sultams.Org Lett. 2011 Oct 7;13(19):5148-51. doi: 10.1021/ol201962n. Epub 2011 Sep 7. Org Lett. 2011. PMID: 21899284 Free PMC article.
-
Synthesis of isochromene-type scaffolds via single-flask Diels-Alder-[4 + 2]-annulation sequence of a silyl-substituted diene with menadione.Org Lett. 2014 Jun 20;16(12):3320-3. doi: 10.1021/ol501329t. Epub 2014 Jun 11. Org Lett. 2014. PMID: 24918110 Free PMC article.
-
Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach.Molecules. 2022 Dec 30;28(1):308. doi: 10.3390/molecules28010308. Molecules. 2022. PMID: 36615500 Free PMC article. Review.
-
Stereoselective C-glycosidations with achiral and enantioenriched allenylsilanes.Org Lett. 2010 Oct 15;12(20):4624-7. doi: 10.1021/ol1019629. Org Lett. 2010. PMID: 20839812 Free PMC article.
References
-
- Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. Wiley; New York: 1984.
- Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. - PubMed
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. - PubMed
- Kelly AR, Wei J, Kesavan S, Marié JC, Windmon N, Young DW, Marcaurelle LA. Org Lett. 2009;11:2257–2260. - PMC - PubMed
- Spiteric C, Moses JE. Angew Chem Int Ed. 2009. p. 48. - DOI
-
- Lewis WG, Green LG, Grynszpan F, Radić Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Angew Chem Int Ed. 2002;41:1053–1057. - PubMed
- Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med Res Rev. 2008;28:278–308. - PubMed
- Lee T, Cho M, Ko SY, Youn HJ, Baek DJ, Cho WJ, Kang CY, Kim S. J Med Chem. 2007;50:585–589. - PubMed
- Maurya SK, Gollapalli DR, Kirubakaran S, Zhang M, Johnson CR, Benjamin NN, Hedstrom L, Cuny GD. J Med Chem. 2009;52:4623–4630. - PMC - PubMed
- Kolb HC, Sharpless KB. Drug Discovery Today. 2003;8:1128–1137. - PubMed
- Puig-Basagoiti F, Qing M, Dong H, Zhang B, Zou G, Yuan Z, Shi PY. Antiviral Research. 2009;83:71–79. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

