Pd(II)-catalyzed enantioselective C-H olefination of diphenylacetic acids
- PMID: 20017549
- PMCID: PMC2806936
- DOI: 10.1021/ja909571z
Pd(II)-catalyzed enantioselective C-H olefination of diphenylacetic acids
Abstract
Pd(II)-catalyzed enantioselective C-H olefination of diphenylacetic acid substrates has been achieved through the use of monoprotected chiral amino acid ligands. The absolute configuration of the resulting olefinated products is consistent with that of a proposed C-H insertion intermediate.
Figures

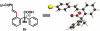
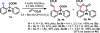
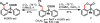
References
-
-
For recent reviews see: Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.. Daugulis O, Zaitsev VG, Shabashov D, Pham Q-N, Lazareva A. Synlett. 2006:3382.. Yu J-Q, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041.. Campeau L-C, Stuart DR, Fagnou K. Aldrichimica Acta. 2007;40:35.. Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117.
-
-
-
For a review on stereoselective C-H functionalization, see: Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242.
-
-
- Shi B-F, Maugel N, Zhang Y-H, Yu J-Q. Angew. Chem., Int. Ed. 2008;47:4761. - PubMed
-
-
For a Ru-catalyzed atropselective alkylation, see: Kakiuchi F, Le Gendre P, Yamada A, Ohtaki H, Murai S. Tetrahedron: Asymmetry. 2000;11:2647.
-
-
-
For a Pd(0)-catalyzed enantioselective arylation see: Albicker MR, Cramer N. Angew. Chem., Int. Ed. 2009;48:9139.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

