Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles
- PMID: 20017632
- PMCID: PMC2798016
- DOI: 10.1086/649228
Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles
Abstract
Background: Morbidity and mortality due to influenza could be reduced by improved vaccination.
Methods: To develop a novel skin delivery method that is simple and allows for easy self-administration, we prepared microneedle patches with stabilized influenza vaccine and investigated their protective immune responses.
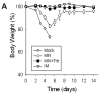
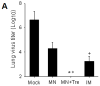
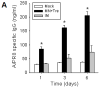
Results: Mice vaccinated with a single microneedle dose of trehalose-stabilized influenza vaccine developed strong antibody responses that were long-lived. Compared with traditional intramuscular vaccination, stabilized microneedle vaccination was superior in inducing protective immunity, as was evidenced by efficient clearance of virus from the lung and enhanced humoral and antibody-secreting cell immune responses after 100% survival from lethal challenge. Vaccine stabilization was found to be important, because mice vaccinated with an unstabilized microneedle vaccine elicited a weaker immunoglobulin G 2a antibody response, compared with the stabilized microneedle vaccine, and were only partially protected against viral challenge. Improved trafficking of dendritic cells to regional lymph nodes as a result of microneedle delivery to the skin might play a role in contributing to improved protective immunity.
Conclusions: These findings suggest that vaccination of the skin using a microneedle patch can improve protective efficacy and induce long-term sustained immunogenicity and may also provide a simple method of administration to improve influenza vaccination coverage.
Conflict of interest statement
Figures









Similar articles
-
Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch.Clin Vaccine Immunol. 2013 Sep;20(9):1433-9. doi: 10.1128/CVI.00251-13. Epub 2013 Jul 17. Clin Vaccine Immunol. 2013. PMID: 23863506 Free PMC article.
-
Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization.J Virol. 2010 Aug;84(15):7760-9. doi: 10.1128/JVI.01849-09. Epub 2010 May 19. J Virol. 2010. PMID: 20484519 Free PMC article.
-
Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin.PLoS One. 2009 Sep 25;4(9):e7152. doi: 10.1371/journal.pone.0007152. PLoS One. 2009. PMID: 19779615 Free PMC article.
-
Microneedle-based vaccines.Curr Top Microbiol Immunol. 2009;333:369-93. doi: 10.1007/978-3-540-92165-3_18. Curr Top Microbiol Immunol. 2009. PMID: 19768415 Free PMC article. Review.
-
Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy.Expert Opin Drug Deliv. 2012 May;9(5):541-50. doi: 10.1517/17425247.2012.676038. Epub 2012 Apr 5. Expert Opin Drug Deliv. 2012. PMID: 22475249 Free PMC article. Review.
Cited by
-
Microneedle Vaccination Elicits Superior Protection and Antibody Response over Intranasal Vaccination against Swine-Origin Influenza A (H1N1) in Mice.PLoS One. 2015 Jun 18;10(6):e0130684. doi: 10.1371/journal.pone.0130684. eCollection 2015. PLoS One. 2015. PMID: 26086590 Free PMC article.
-
Microneedle Coating Methods: A Review with a Perspective.J Pharmacol Exp Ther. 2019 Sep;370(3):555-569. doi: 10.1124/jpet.119.258707. Epub 2019 Jun 7. J Pharmacol Exp Ther. 2019. PMID: 31175217 Free PMC article. Review.
-
The success of microneedle-mediated vaccine delivery into skin.Hum Vaccin Immunother. 2016 Nov;12(11):2975-2983. doi: 10.1080/21645515.2016.1171440. Epub 2016 Apr 6. Hum Vaccin Immunother. 2016. PMID: 27050528 Free PMC article. Review.
-
Syringe free vaccination with CAF01 Adjuvated Ag85B-ESAT-6 in Bioneedles provides strong and prolonged protection against tuberculosis.PLoS One. 2010 Nov 29;5(11):e15043. doi: 10.1371/journal.pone.0015043. PLoS One. 2010. PMID: 21124731 Free PMC article.
-
Microneedles for drug and vaccine delivery.Adv Drug Deliv Rev. 2012 Nov;64(14):1547-68. doi: 10.1016/j.addr.2012.04.005. Epub 2012 May 1. Adv Drug Deliv Rev. 2012. PMID: 22575858 Free PMC article. Review.
References
-
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: Immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scandinavian J Immunol. 2004;59:1–15. - PubMed
-
- Palese P. Influenza: Old and new threats. Nat Med. 2004;10:S82–7. - PubMed
-
- Centers for Disease Control and Prevention. Prevention and control of influenza, recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2008;57:1–60. - PubMed
-
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. - PubMed
-
- Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

