Characterizing functional stem cell-cardiomyocyte interactions
- PMID: 20017697
- PMCID: PMC2819199
- DOI: 10.2217/rme.09.69
Characterizing functional stem cell-cardiomyocyte interactions
Abstract
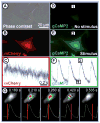
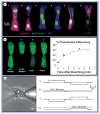
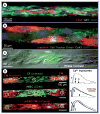
Despite the progress in traditional pharmacological and organ transplantation therapies, heart failure still afflicts 5.3 million Americans. Since June 2000, stem cell-based approaches for the prevention and treatment of heart failure have been pursued in clinics with great excitement; however, the exact mechanisms of how transplanted cells improve heart function remain elusive. One of the main difficulties in answering these questions is the limited ability to directly access and study interactions between implanted cells and host cardiomyocytes in situ. With the growing number of candidate cell types for potential clinical use, it is becoming increasingly more important to establish standardized, well-controlled in vitro and in situ assays to compare the efficacy and safety of different stem cells in cardiac repair. This article describes recent innovative methodologies to characterize direct functional interactions between stem cells and cardiomyocytes, aimed to facilitate the rational design of future cell-based therapies for heart disease.
Figures




References
-
- Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics—2008 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. - PubMed
-
- Marelli D, Desrosiers C, El-Alfy M, Kao RL, Chiu RC. Cell transplantation for myocardial repair: an experimental approach. Cell Transplant. 1992;1(6):383–390. - PubMed
-
- Menasche P, Alfieri O, Janssens S, et al. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial. First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
