Causes of dysregulation of lipid metabolism in chronic renal failure
- PMID: 20017835
- PMCID: PMC2874323
- DOI: 10.1111/j.1525-139X.2009.00661.x
Causes of dysregulation of lipid metabolism in chronic renal failure
Abstract
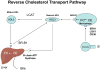
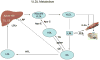
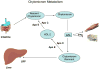
End-stage renal disease (ESRD) is associated with accelerated atherosclerosis and premature death from cardiovascular disease. These events are driven by oxidative stress inflammation and lipid disorders. ESRD-induced lipid abnormalities primarily stem from dysregulation of high-density lipoprotein (HDL), triglyceride-rich lipoprotein metabolism, and oxidative modification of lipoproteins. In this context, production and plasma concentration of Apo-I and Apo-II are reduced, HDL maturation is impaired, HDL composition is altered, HDL antioxidant and anti-inflammatory functions are depressed, clearance of triglyceride-rich lipoproteins and their atherogenic remnants is impaired, their composition is altered, and their plasma concentration is elevated in ESRD. The associated defect in HDL maturation is largely caused by acquired lecithin-cholesterol acyltransferase deficiency while its triglyceride enrichment is due to hepatic lipase deficiency. Hypertriglyceridemia, abnormal composition, and impaired clearance of triglyceride-rich lipoproteins and their remnants are mediated by down-regulation of lipoprotein lipase, hepatic lipase, very low-density lipoprotein (VLDL) receptor, and LDL receptor-related protein, relative reduction in ApoC-II/ApoC-III ratio, up-regulation of acyl-CoA cholesterol acyltransferase, and elevated plasma level of cholesterol ester-poor prebeta HDL. Impaired clearance and accumulation of oxidation-prone VLDL and chylomicron remnants and abnormal LDL composition in the face of oxidative stress and inflammation favors their uptake by macrophages and resident cells in the artery wall. The effect of heightened influx of lipids is compounded by impaired HDL-mediated reverse cholesterol transport leading to foam cell formation which is the central event in atherosclerosis plaque formation and subsequent plaque rupture, thrombosis, and tissue damage.
Figures
Similar articles
-
Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences.Am J Physiol Renal Physiol. 2006 Feb;290(2):F262-72. doi: 10.1152/ajprenal.00099.2005. Am J Physiol Renal Physiol. 2006. PMID: 16403839 Review.
-
Abnormalities in uremic lipoprotein metabolism and its impact on cardiovascular disease.Am J Kidney Dis. 2001 Oct;38(4 Suppl 1):S14-9. doi: 10.1053/ajkd.2001.27384. Am J Kidney Dis. 2001. PMID: 11576915 Review.
-
HDL metabolism and activity in chronic kidney disease.Nat Rev Nephrol. 2010 May;6(5):287-96. doi: 10.1038/nrneph.2010.36. Epub 2010 Mar 23. Nat Rev Nephrol. 2010. PMID: 20308998 Review.
-
Lipid disorders and their relevance to outcomes in chronic kidney disease.Blood Purif. 2011;31(1-3):189-96. doi: 10.1159/000321845. Epub 2011 Jan 10. Blood Purif. 2011. PMID: 21228589 Review.
-
Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease.J Ren Nutr. 2010 Sep;20(5 Suppl):S35-43. doi: 10.1053/j.jrn.2010.05.010. J Ren Nutr. 2010. PMID: 20797569 Review.
Cited by
-
Discordant associations of lipid parameters with albuminuria and chronic kidney disease: a population-based study.Lipids Health Dis. 2015 Nov 25;14:152. doi: 10.1186/s12944-015-0153-8. Lipids Health Dis. 2015. PMID: 26607500 Free PMC article.
-
The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease.Int J Mol Sci. 2020 Jan 17;21(2):601. doi: 10.3390/ijms21020601. Int J Mol Sci. 2020. PMID: 31963445 Free PMC article. Review.
-
Estimating the Level of Carbamoylated Plasma Non-High-Density Lipoproteins Using Infrared Spectroscopy.J Clin Med. 2019 May 31;8(6):774. doi: 10.3390/jcm8060774. J Clin Med. 2019. PMID: 31159214 Free PMC article.
-
High-density lipoprotein in uremic patients: metabolism, impairment, and therapy.Int Urol Nephrol. 2014 Jan;46(1):27-39. doi: 10.1007/s11255-012-0366-y. Epub 2013 Feb 27. Int Urol Nephrol. 2014. PMID: 23443874 Review.
-
Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients.Vasc Health Risk Manag. 2013;9:747-61. doi: 10.2147/VHRM.S51710. Epub 2013 Nov 28. Vasc Health Risk Manag. 2013. PMID: 24348043 Free PMC article. Clinical Trial.
References
-
- Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, Matas A, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li S, Li S, Roberts T, Snyder J, Solid C, Wang C, Weinhandl E, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Johnson R, Sheets D, Forrest B, Berrini D, Constantini E, Everson S, Frederick P, Eggers P, Agodoa L United States Renal Data System. Excerpts from the United States Renal Data System 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2005;45(1 Suppl 1):A5–7. S1–280. - PubMed
-
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. - PubMed
-
- McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol. 2003;41:725–728. - PubMed
-
- Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. - PubMed
-
- Vaziri ND. Effect of chronic renal failure on nitric oxide metabolism. Am J Kidney Dis. 2001;38:S74–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

