A novel anti-mycobacterial function of mitogen-activated protein kinase phosphatase-1
- PMID: 20017901
- PMCID: PMC2804704
- DOI: 10.1186/1471-2172-10-64
A novel anti-mycobacterial function of mitogen-activated protein kinase phosphatase-1
Abstract
Background: Mycobacterium tuberculosis (MTB) is a major cause of morbidity and mortality in the world. To combat against this pathogen, immune cells release cytokines including tumor necrosis factor-alpha (TNF-alpha), which is pivotal in the development of protective granulomas. Our previous results showed that Bacillus Calmette Guerin (BCG), a mycobacterium used as a model to investigate the immune response against MTB, stimulates the induction of TNF-alpha via mitogen-activated protein kinase (MAPK) in human blood monocytes. Since MAPK phosphatase-1 (MKP-1) is known to regulate MAPK activities, we examined whether MKP-1 plays a role in BCG-induced MAPK activation and cytokine expression.
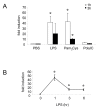
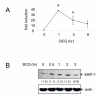
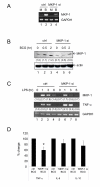
Results: Primary human blood monocytes were treated with BCG and assayed for MKP-1 expression. Our results demonstrated that following exposure to BCG, there was an increase in the expression of MKP-1. Additionally, the induction of MKP-1 was regulated by p38 MAPK and extracellular signal-regulated kinase 1 and 2 (ERK1/2). Surprisingly, when MKP-1 expression was blocked by its specific siRNA, there was a significant decrease in the levels of phospho-MAPK (p38 MAPK and ERK1/2) and TNF-alpha inducible by BCG.
Conclusions: Since TNF-alpha is pivotal in granuloma formation, the results indicated an unexpected positive function of MKP-1 against mycobacterial infection as opposed to its usual phosphatase activity.
Figures
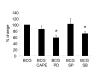

Similar articles
-
Heat shock inhibition of lipopolysaccharide-mediated tumor necrosis factor expression is associated with nuclear induction of MKP-1 and inhibition of mitogen-activated protein kinase activation.Crit Care Med. 2004 Nov;32(11):2284-92. doi: 10.1097/01.ccm.0000145580.96994.c9. Crit Care Med. 2004. PMID: 15640643
-
The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages.J Biol Chem. 2004 Dec 24;279(52):54023-31. doi: 10.1074/jbc.M408444200. Epub 2004 Oct 13. J Biol Chem. 2004. PMID: 15485842
-
Activation of ERK1/2 and TNF-alpha production are mediated by calcium/calmodulin, and PKA signaling pathways during Mycobacterium bovis infection.J Infect. 2006 Feb;52(2):147-53. doi: 10.1016/j.jinf.2005.02.027. J Infect. 2006. PMID: 16442440
-
MAP kinase phosphatase-1, a gatekeeper of the acute innate immune response.Life Sci. 2020 Jan 15;241:117157. doi: 10.1016/j.lfs.2019.117157. Epub 2019 Dec 16. Life Sci. 2020. PMID: 31837332 Free PMC article. Review.
-
Mitogen-Activated Protein Kinase Phosphatase (MKP)-1 in Nervous System Development and Disease.Mol Neurobiol. 2015;51(3):1158-67. doi: 10.1007/s12035-014-8786-6. Epub 2014 Jun 24. Mol Neurobiol. 2015. PMID: 24957007 Review.
Cited by
-
Expression of inhibitory regulators of innate immunity in patients with active tuberculosis.BMC Infect Dis. 2015 Feb 26;15:98. doi: 10.1186/s12879-015-0833-z. BMC Infect Dis. 2015. PMID: 25887604 Free PMC article.
-
A role for c-Myc in regulating anti-mycobacterial responses.Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17749-54. doi: 10.1073/pnas.1104892108. Epub 2011 Oct 12. Proc Natl Acad Sci U S A. 2011. PMID: 21997212 Free PMC article.
-
Chronic TNF exposure induces glucocorticoid-like immunosuppression in the alveolar macrophages of aged mice that enhances their susceptibility to pneumonia.Aging Cell. 2024 Jun;23(6):e14133. doi: 10.1111/acel.14133. Epub 2024 Mar 8. Aging Cell. 2024. PMID: 38459711 Free PMC article.
-
Mitogen-activated protein kinases mediate Mycobacterium tuberculosis-induced CD44 surface expression in monocytes.J Biosci. 2012 Mar;37(1):41-54. doi: 10.1007/s12038-011-9179-x. J Biosci. 2012. PMID: 22357202
-
A role for interleukin-17A in modulating intracellular survival of Mycobacterium bovis bacillus Calmette-Guérin in murine macrophages.Immunology. 2013 Nov;140(3):323-34. doi: 10.1111/imm.12140. Immunology. 2013. PMID: 23808492 Free PMC article.
References
-
- World Health Organization. Global tuberculosis control 2008 - surveillance, planning, financing. Geneva: WHO Press; 2008.
-
- Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/A:1023309928879. - DOI - PubMed
-
- Cheung BK, Lee DC, Li JC, Lau YL, Lau AS. A role for double-stranded RNA-activated protein kinase PKR in Mycobacterium-induced cytokine expression. J Immunol. 2005;175:7218–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

