DDIT3/CHOP and the sarcoma fusion oncoprotein FUS-DDIT3/TLS-CHOP bind cyclin-dependent kinase 2
- PMID: 20017906
- PMCID: PMC2804592
- DOI: 10.1186/1471-2121-10-89
DDIT3/CHOP and the sarcoma fusion oncoprotein FUS-DDIT3/TLS-CHOP bind cyclin-dependent kinase 2
Abstract
Background: The DDIT3 gene encodes a transcription factor belonging to the CCAAT/enhancer binding protein (C/EBP) family. It is normally expressed at very low levels but is activated by cellular stress conditions and induces G1 arrest and, in some cell types, apoptosis. DDIT3 is found as a part of the fusion oncogene FUS-DDIT3 that is causal for the development of myxoid/round-cell liposarcomas (MLS/RCLS).
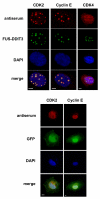
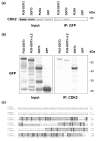
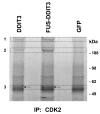
Results: In the present study, we searched for putative interaction partners of DDIT3 and the oncogenic FUS-DDIT3 among G1 cyclins and cyclin-dependent kinases. We found that FUS-DDIT3 and the normal DDIT3 bind CDK2. In addition, CDK2 showed an increased affinity for cytoskeletal proteins in cells expressing FUS-DDIT3 and DDIT3.
Conclusions: We conclude that DDIT3 binds CDK2 and that many of the observed biological effects of DDIT3 may involve interaction with CDK2.
Figures

Similar articles
-
Nuclear expression of FLT1 and its ligand PGF in FUS-DDIT3 carrying myxoid liposarcomas suggests the existence of an intracrine signaling loop.BMC Cancer. 2010 Jun 1;10:249. doi: 10.1186/1471-2407-10-249. BMC Cancer. 2010. PMID: 20515481 Free PMC article.
-
The myxoid liposarcoma FUS-DDIT3 fusion oncoprotein deregulates NF-kappaB target genes by interaction with NFKBIZ.Oncogene. 2009 Jan 15;28(2):270-8. doi: 10.1038/onc.2008.378. Epub 2008 Oct 13. Oncogene. 2009. PMID: 18850010
-
The myxoid/round cell liposarcoma fusion oncogene FUS-DDIT3 and the normal DDIT3 induce a liposarcoma phenotype in transfected human fibrosarcoma cells.Am J Pathol. 2006 May;168(5):1642-53. doi: 10.2353/ajpath.2006.050872. Am J Pathol. 2006. PMID: 16651630 Free PMC article.
-
FUS::DDIT3 Fusion Protein in the Development of Myxoid Liposarcoma and Possible Implications for Therapy.Biomolecules. 2024 Oct 14;14(10):1297. doi: 10.3390/biom14101297. Biomolecules. 2024. PMID: 39456230 Free PMC article. Review.
-
Gene of the month: DDIT3.J Clin Pathol. 2024 Mar 20;77(4):211-216. doi: 10.1136/jcp-2023-208963. J Clin Pathol. 2024. PMID: 38053287 Review.
Cited by
-
New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer.Cell Cycle. 2010 Aug 1;9(15):2986-95. doi: 10.4161/cc.9.15.12515. Epub 2010 Aug 28. Cell Cycle. 2010. PMID: 20647765 Free PMC article. Review.
-
Effect of dietary restriction and subsequent re-alimentation on the transcriptional profile of hepatic tissue in cattle.BMC Genomics. 2016 Mar 17;17:244. doi: 10.1186/s12864-016-2578-5. BMC Genomics. 2016. PMID: 26984536 Free PMC article.
-
Investigating the mechanism of hepatocellular carcinoma progression by constructing genetic and epigenetic networks using NGS data identification and big database mining method.Oncotarget. 2016 Nov 29;7(48):79453-79473. doi: 10.18632/oncotarget.13100. Oncotarget. 2016. PMID: 27821810 Free PMC article.
-
Defining the antigen receptor-dependent regulatory network that induces arrest of cycling immature B-lymphocytes.BMC Syst Biol. 2010 Dec 9;4:169. doi: 10.1186/1752-0509-4-169. BMC Syst Biol. 2010. PMID: 21143896 Free PMC article.
-
Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy.PLoS One. 2011;6(6):e21834. doi: 10.1371/journal.pone.0021834. Epub 2011 Jun 30. PLoS One. 2011. PMID: 21738796 Free PMC article.
References
-
- Sylvester SL, ap Rhys CM, Luethy-Martindale JD, Holbrook NJ. Induction of GADD153, a CCAAT/enhancer-binding protein (C/EBP)-related gene, during the acute phase response in rats. Evidence for the involvement of C/EBPs in regulating its expression. J Biol Chem. 1994;269(31):20119–20125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

