The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2)
- PMID: 20017911
- PMCID: PMC2809058
- DOI: 10.1186/1741-7007-7-87
The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2)
Abstract
Background: Obesity is a chronic low inflammatory state. In the obesity condition the white adipose tissue (WAT) is massively infiltrated with monocytes/macrophages, and the nature of the signals recruiting these inflammatory cells has yet to be fully elucidated. Haptoglobin (Hp) is an inflammatory marker and its expression is induced in the WAT of obese subjects. In an effort to elucidate the biological significance of Hp presence in the WAT and of its upregulation in obesity we formulated the hypothesis that Hp may serve as a macrophage chemoattractant.
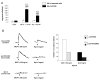
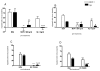
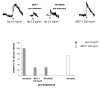
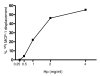
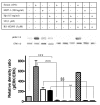
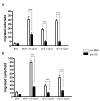
Results: We demonstrated by chemotaxis assay that Hp is able to attract chemokine (C-C motif) receptor 2 (CCR2)-transfected pre-B lymphocytes and monocytes in a dose-dependent manner. Moreover, Hp-mediated migration of monocytes is impaired by CCR2-specific inhibition or previous cell exposure to monocyte chemoattractant protein 1 (MCP1) (also known as CCR2 ligand or chemokine (C-C motif) ligand 2 (CCL2)). Downstream effects of Hp/CCR2 interaction were also investigated: flow cytometry proved that monocytes treated with Hp show reduced CCR2 expression on their surface; Hp interaction induces calcium release that is reduced upon pretreatment with CCR2 antagonist; extracellular signal-regulated kinase (ERK)1/2, a signal transducer activated by CCR2, is phosphorylated following Hp treatment and this phosphorylation is reduced when cells are pretreated with a specific CCR2 inhibitor. Consistently, blocking the ERK1/2 pathway with U0126, the selective inhibitor of the ERK upstream mitogen-activated protein (MAP)-ERK kinase (MEK), results in a dramatic reduction (by almost 100%) of the capability of Hp to induce monocyte migration.
Conclusions: Our data show that Hp is a novel monocyte chemoattractant and that its chemotactic potential is mediated, at least in part. by its interaction with CCR2.
Figures

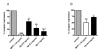
References
-
- de Kleijn DP, Smeets MB, Kemmeren PP, Lim SK, Van Middelaar BJ, Velema E, Schoneveld A, Pasterkamp G, Borst C. Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J. 2002;16:1123–1125. - PubMed
-
- Chiellini C, Bertacca A, Novelli SE, Görgün CZ, Ciccarone A, Giordano A, Xu H, Soukas A, Costa M, Gandini D, Dimitri R, Bottone P, Cecchetti P, Pardini E, Perego L, Navalesi R, Folli F, Benzi L, Cinti S, Friedman JM, Hotamisligil GS, Maffei M. Obesity modulates the expression of haptoglobin in the white adipose tissue via TNFalpha. J Cell Physiol. 2002;190:251–258. doi: 10.1002/jcp.10061. - DOI - PubMed
-
- Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, López-Soriano J, Centoni R, Ciccarone AM, Benzi L, Vitti P, Del Prato S, Pinchera A, Maffei M. Serum haptoglobin: a novel marker of adiposity in humans. J Clin Endocrinol Metab. 2004;89:2678–2683. doi: 10.1210/jc.2003-031965. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

