Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study
- PMID: 20017918
- PMCID: PMC2811100
- DOI: 10.1186/1758-2652-12-38
Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study
Abstract
Background: Clinical, immunologic and virologic outcomes at large HIV/AIDS care clinics in resource poor settings are poorly described beyond the first year of highly active antiretroviral treatment (HAART). We aimed to prospectively evaluate long-term treatment outcomes at a large scale HIV/AIDS care clinic in South Africa.
Methods: Cohort study of patients initiating HAART between April 1, 2004 and March 13, 2007, and followed up until April 1, 2008 at a public HIV/AIDS care clinic in Johannesburg, South Africa. We performed time to event analysis on key treatment outcomes and program impact parameters including mortality, retention in care, CD4 count gain, virologic success and first line regimen durability.
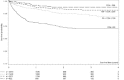
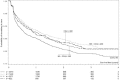
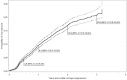
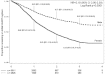
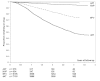
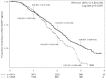
Results: 7583 HIV-infected patients initiated care and contributed to 161,000 person months follow up. Overall mortality rate was low (2.9 deaths per 100 person years, 95% CI 2.6-3.2), but high in the first three months of HAART (8.4 per 100 person years, 95% CI 7.2-9.9). Long-term on-site retention in care was relatively high (74.4% at 4 years, 95%CI 73.2-75.6). CD4 count was above 200 cells/mm(3 )after 6 months of treatment in almost all patients. By the fourth year of HAART, the majority (59.6%, 95%CI 57.8-61.4) of patients had at least one first line drug (mainly stavudine) substituted. Women were twice as likely to experience drug substitution (OR 1.97, 95% CI 1.80-2.16). By 6 months of HAART, 90.8% suppressed virus below 400 copies. Among those with initial viral suppression, 9.4% (95% CI 8.5-10.3%) had viral rebound within one year of viral suppression, 16.8% (95% CI 15.5-18.1) within 2 years, and 20.6% (95% CI 18.9-22.4) within 3 years of initial suppression. Only 10% of women and 13% of men initiated second line HAART.
Conclusion: Despite advanced disease presentation and a very large-scale program, high quality care was achieved as indicated by good long-term clinical, immunologic and virologic outcomes and a low rate of second line HAART initiation. High rates of single drug substitution suggest that the public health approach to HAART could be further improved by the use of a more durable first line regimen.
Figures
References
-
- UNAIDS/WHO. AIDS Epidemic Update. Geneva. 2007. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive...
-
- HIV and AIDS and STI Strategic Plan for South Africa, 2007-2011
-
- Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;12(4):315–320. - PubMed
-
- Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, Karungi G, Szumilin E, Balandine S, Fedida G. et al.Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;12(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

