Adolescent type 1 Diabetes Cardio-renal Intervention Trial (AdDIT)
- PMID: 20017932
- PMCID: PMC2814806
- DOI: 10.1186/1471-2431-9-79
Adolescent type 1 Diabetes Cardio-renal Intervention Trial (AdDIT)
Abstract
Background: The prognosis for young people diagnosed with diabetes during childhood remains poor and this is mainly related to the long-term risk of developing vascular complications.Microalbuminuria identifies subjects at risk for diabetic nephropathy (DN) and cardiovascular disease (CVD). It is often detected in adolescence but is rarely treated before the age of 18 years, as at the end of puberty albumin excretion may decline and in some subjects will return into the normal range. However, evidence indicates that subjects with both transient and persistent microalbuminuria have experienced renal damage during puberty and thus reno-protection to prevent long-term complications is warranted. In adults with diabetes and microalbuminuria, the use of angiotensin converting enzyme inhibitors (ACEI) and Statins is increasing, and in order to determine whether these agents are of value in the adolescent population a large randomized controlled clinical trial is needed.
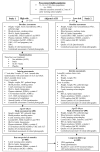
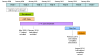
Methods/design: The Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT) is a multi-center, randomized, double-blind, placebo-controlled trial of ACEI and Statin therapy in adolescents with type 1 diabetes. 500 high-risk adolescents, defined on the basis of their albumin excretion, are randomized to receive either ACEI (Quinapril) or Statins (Atorvastatin) or combination therapy or placebo for 3-4 years. There will also be a parallel open observational study, based on the follow-up of 400 low-risk non-randomized adolescents. The major endpoint of the study is the change in albumin excretion; secondary endpoints include markers of CVD, renal function, retinopathy, quality of life combined with assessment of compliance and potential health economic benefits.
Discussion: AdDIT will provide important data on the potential renal and cardiovascular protective effects of ACEI and Statins in high-risk adolescents. Long-term follow-up of the randomized subjects will provide direct evidence of disease outcomes, in addition to the data on early surrogate measures of DN and CVD. Follow-up of non-randomized low-risk subjects will determine the potential impact of intervention on DN and CVD. AdDIT will help to determine whether, in addition to encouraging young people to achieve good glycaemic control, pharmacological cardio-renal protection should also be implemented. EUDRACT NUMBER: 2007-001039-72
Trial registration number: ISRCTN91419926.
Figures
References
-
- Orchard TJ. From diagnosis and classification to complications and therapy. DCCT. Part II? Diabetes Control and Complications Trial. Diabetes care. 1994;17(4):326–338. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical