Comparison of the effects of vitamin D products in a psoriasis plaque test and a murine psoriasis xenograft model
- PMID: 20017943
- PMCID: PMC2804591
- DOI: 10.1186/1479-5876-7-107
Comparison of the effects of vitamin D products in a psoriasis plaque test and a murine psoriasis xenograft model
Abstract
The aim of the present study was to compare the effects of Daivobet and calcipotriol on clinical score and biomarker responses in a modified version of the Scholtz-Dumas psoriasis plaque assay. Furthermore, it was the aim to compare the effects of calcipotriol and betamethasone in the murine psoriasis xenograft model. Twenty four patients with psoriasis were treated topically once daily for three weeks, whereas the grafted mice were treated for four weeks. Clinical responses were scored twice weekly and biopsies were taken at the end of each study to analyse for skin biomarkers by histology and immunohistochemistry. The results clearly demonstrate effects on both clinical signs and biomarkers. In the patient study the total clinical score was reduced significantly with both Daivobet and calcipotriol. Both treatments reduced epidermal thickness, Ki-67 and cytokeratin 16 expression. T cell infiltration was significantly reduced by Daivobet but only marginally by calcipotriol. Both treatments showed strong effects on the epidermal psoriatic phenotype.Results from the xenograft model essentially showed the same results. However differences were observed when investigating subtypes of T cells.The study demonstrates the feasibility of obtaining robust biomarker data in the psoriasis plaque test that correlate well with those obtained in other clinical studies. Furthermore, the biomarker data from the plaque test correlate with biopsy data from the grafted mice.
Figures
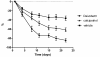
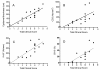


References
-
- Dumas KJ, Scholtz JR. The psoriasis bio-assay for topical corticosteroid activity. Acta Derm Venereol. 1972;52(1):43–48. - PubMed
-
- Villadsen LS, Schuurman J, Beurskens F, Dam TN, Dagnaes-Hansen F, Skov L, Rygaard J, Voorhorst-Ogink MM, Gerritsen AF, van Dijk MA, Parren PW, Baadsgaard O, Winkel JG van de. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112(10):1571–1580. - PMC - PubMed
-
- Kragballe K, Noerrelund KL, Lui H, Ortonne JP, Wozel G, Uurasmaa T, Fleming C, Estebaranz JL, Hanssen LI, Persson LM. Efficacy of once-daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol. 2004;150(6):1167–1173. doi: 10.1111/j.1365-2133.2004.05986.x. - DOI - PubMed
-
- Guenther L, Kerkhof PC van de, Snellman E, Kragballe K, Chu AC, Tegner E, Garcia-Diez A, Springborg J. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol. 2002;147(2):316–323. doi: 10.1046/j.1365-2133.2002.04967.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

