Characterization of the stem cell system of the acoel Isodiametra pulchra
- PMID: 20017953
- PMCID: PMC2806412
- DOI: 10.1186/1471-213X-9-69
Characterization of the stem cell system of the acoel Isodiametra pulchra
Abstract
Background: Tissue plasticity and a substantial regeneration capacity based on stem cells are the hallmark of several invertebrate groups such as sponges, cnidarians and Platyhelminthes. Traditionally, Acoela were seen as an early branching clade within the Platyhelminthes, but became recently positioned at the base of the Bilateria. However, little is known on how the stem cell system in this new phylum is organized. In this study, we wanted to examine if Acoela possess a neoblast-like stem cell system that is responsible for development, growth, homeostasis and regeneration.
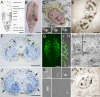
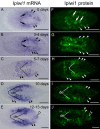
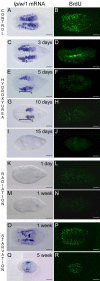
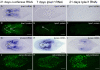
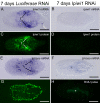
Results: We established enduring laboratory cultures of the acoel Isodiametra pulchra (Acoela, Acoelomorpha) and implemented in situ hybridization and RNA interference (RNAi) for this species. We used BrdU labelling, morphology, ultrastructure and molecular tools to illuminate the morphology, distribution and plasticity of acoel stem cells under different developmental conditions. We demonstrate that neoblasts are the only proliferating cells which are solely mesodermally located within the organism. By means of in situ hybridisation and protein localisation we could demonstrate that the piwi-like gene ipiwi1 is expressed in testes, ovaries as well as in a subpopulation of somatic stem cells. In addition, we show that germ cell progenitors are present in freshly hatched worms, suggesting an embryonic formation of the germline. We identified a potent stem cell system that is responsible for development, homeostasis, regeneration and regrowth upon starvation.
Conclusions: We introduce the acoel Isodiametra pulchra as potential new model organism, suitable to address developmental questions in this understudied phylum. We show that neoblasts in I. pulchra are crucial for tissue homeostasis, development and regeneration. Notably, epidermal cells were found to be renewed exclusively from parenchymally located stem cells, a situation known only from rhabditophoran flatworms so far. For further comparison, it will be important to analyse the stem cell systems of other key-positioned understudied taxa.
Figures


References
-
- Ladurner P, Egger B, De Mulder K, Pfister D, Kuales G, Salvenmoser W. In: stem cells: from hydra to man. 1. Bosch TC, editor. Vol. 1. Springer; 2008. The stem cell system of the basal flatworm Macrostomum lignano; pp. 75–94.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

