Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study
- PMID: 20017955
- PMCID: PMC2803172
- DOI: 10.1186/1465-9921-10-126
Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study
Abstract
Background: Toll-like receptor 4 (TLR4) is essential in lipopolysaccharide (LPS)-induced fibroblast activation and collagen secretion in vitro. However, its effects on the process of lung fibroblast activation and fibrosis initiation during LPS induced acute lung injury (ALI) remain unknown. The goal of the present study was to determine the effect of inhibiting TLR4 on LPS-induced ALI and fibrosis in vivo.
Methods: The ALI model was established by intraperitoneal injection of LPS in mice. TLR4-small hairpin RNA (shRNA) lentivirus was injected intravenously into the mice to inhibit TLR4 expression. mRNA and protein levels were detected by real-time PCR and Western-blot analysis, respectively. The contents of the C-terminal propeptide of type I procollagen (PICP) in bronchoalveolar lavage fluid (BALF) were detected by ELISA, and the degree of fibrosis was detected by van Gieson collagen staining, the hydroxyproline assay, and alpha smooth muscle actin (alpha-SMA) immunohistochemical staining.
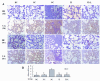
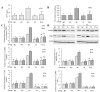
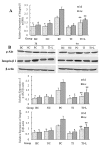
Results: Overexpression of TLR4, type I procollagen, alpha-SMA, and p-AKT in murine pulmonary tissue after intraperitoneal injection of LPS at 72 hours and 28 days were detected. Moreover, the degree of fibrosis was shown to increase by ELISA analysis of PICP in BALF, van Gieson collagen staining, the hydroxyproline assay, and alpha-SMA immunohistochemical staining. All of these changes were alleviated by intravenous infection with TLR4-shRNA lentivirus.
Conclusions: Inhibiting TLR4 signaling could ameliorate fibrosis at the early stage of ALI induced by LPS.
Figures

References
-
- Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. American journal of respiratory and critical care medicine. 2000;162(5):1783–1788. - PubMed
-
- Janardhan KS, McIsaac M, Fowlie J, Shrivastav A, Caldwell S, Sharma RK, Singh B. Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histology and histopathology. 2006;21(7):687–696. - PubMed
-
- Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M. et al.CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. The American journal of pathology. 2008;172(6):1650–1663. doi: 10.2353/ajpath.2008.071049. - DOI - PMC - PubMed
-
- Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, Poole AR, Millar AB. Changes in collagen turnover in early acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1999;160(6):1910–1915. - PubMed
-
- Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, Matthay MA. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol. 2004;172(4):2668–2677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

