Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B (Bbeta2)
- PMID: 20017961
- PMCID: PMC2810296
- DOI: 10.1186/1471-2121-10-91
Oxidative stress promotes autophagic cell death in human neuroblastoma cells with ectopic transfer of mitochondrial PPP2R2B (Bbeta2)
Abstract
Background: The multifunctional protein phosphatase 2A (PP2A) is a heterotrimeric serine/threonine protein phosphatase composed of a scaffolding, catalytic and regulatory subunits. By modifying various downstream signal transducers, the aberrant expression of the brain-targeted regulatory subunit PPP2R2B is associated with the onset of a panel of neuronal disorders. The alternatively splicing of PPP2R2B encodes two regulatory subunit isoforms that determine cellular distribution of the neuron-specific holoenzyme to mitochondria (Bbeta2) and cytoplasm (Bbeta1), respectively.
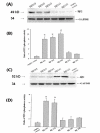
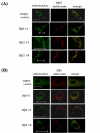
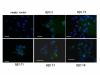
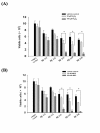
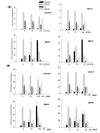
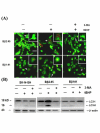
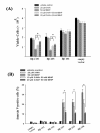
Results: Human neuroblastoma cells were transfected with PPP2R2B constructs encoding the complete sequences of Bbeta2 and Bbeta1, respectively. The colonies with antibiotic resistance were selected as stable cell lines. Both ectopic Bbeta1 and Bbeta2 clones exhibited characteristics of autophagy. To test how cells respond to reactive oxygen species generators, the cells were treated with either hydrogen peroxide or t-butyl hydroperoxide and Bbeta2 clones induced cell death. Suppression of autophagy using either RNA interference of the essential autophagy gene or pharmacological inhibitor rescued cell death caused by oxidative stress.
Conclusions: Cells with ectopically expressed mitochondria-targeted regulatory subunit PPP2R2B of the holoenzyme PP2A were shown predisposed to autophagy and oxidative stress induced cell death that is related to apoptosis. The results promised a model for studying the mechanism and function of aberrant PPP2R2B expression in neuronal cells. The work provided a new target for understanding and prevention of neuropathogenesis.
Figures

Similar articles
-
Differential autophagic cell death under stress with ectopic cytoplasmic and mitochondrial-specific PPP2R2B in human neuroblastoma cells.Apoptosis. 2013 May;18(5):627-38. doi: 10.1007/s10495-013-0809-7. Apoptosis. 2013. PMID: 23381641
-
N-terminal phosphorylation of protein phosphatase 2A/Bβ2 regulates translocation to mitochondria, dynamin-related protein 1 dephosphorylation, and neuronal survival.FEBS J. 2013 Jan;280(2):662-73. doi: 10.1111/j.1742-4658.2012.08631.x. Epub 2012 Jun 8. FEBS J. 2013. PMID: 22583914 Free PMC article.
-
The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission.J Biol Chem. 2008 Dec 26;283(52):36241-8. doi: 10.1074/jbc.M800989200. Epub 2008 Oct 21. J Biol Chem. 2008. PMID: 18940801 Free PMC article.
-
The PP2A subunit PR130 is a key regulator of cell development and oncogenic transformation.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188453. doi: 10.1016/j.bbcan.2020.188453. Epub 2020 Oct 15. Biochim Biophys Acta Rev Cancer. 2020. PMID: 33068647 Review.
-
Protein Phosphatase 2A: a Double-Faced Phosphatase of Cellular System and Its Role in Neurodegenerative Disorders.Mol Neurobiol. 2018 Feb;55(2):1750-1761. doi: 10.1007/s12035-017-0444-3. Epub 2017 Feb 21. Mol Neurobiol. 2018. PMID: 28224476 Review.
Cited by
-
The B55α-containing PP2A holoenzyme dephosphorylates FOXO1 in islet β-cells under oxidative stress.Biochem J. 2012 Jun 1;444(2):239-47. doi: 10.1042/BJ20111606. Biochem J. 2012. PMID: 22417654 Free PMC article.
-
Cell death and autophagy under oxidative stress: roles of poly(ADP-Ribose) polymerases and Ca(2+).Mol Cell Biol. 2012 Sep;32(17):3541-53. doi: 10.1128/MCB.00437-12. Epub 2012 Jul 2. Mol Cell Biol. 2012. PMID: 22751932 Free PMC article.
-
Mitochondrial dysfunction and oxidative stress contribute to the pathogenesis of spinocerebellar ataxia type 12 (SCA12).J Biol Chem. 2011 Jun 17;286(24):21742-54. doi: 10.1074/jbc.M110.160697. Epub 2011 Apr 6. J Biol Chem. 2011. PMID: 21471219 Free PMC article.
-
Protein phosphatase 2A plays an important role in migration of bone marrow stroma cells.Mol Cell Biochem. 2016 Jan;412(1-2):173-80. doi: 10.1007/s11010-015-2624-7. Epub 2015 Dec 26. Mol Cell Biochem. 2016. PMID: 26708215
-
Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD.Arch Biochem Biophys. 2010 Aug 15;500(2):203-9. doi: 10.1016/j.abb.2010.05.013. Epub 2010 May 20. Arch Biochem Biophys. 2010. PMID: 20493163 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

