(D)-Amino acid analogues of DT-2 as highly selective and superior inhibitors of cGMP-dependent protein kinase Ialpha
- PMID: 20018259
- PMCID: PMC2822062
- DOI: 10.1016/j.bbapap.2009.12.004
(D)-Amino acid analogues of DT-2 as highly selective and superior inhibitors of cGMP-dependent protein kinase Ialpha
Abstract
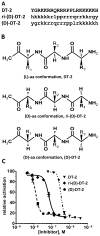
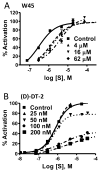
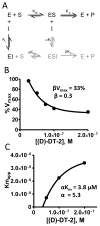
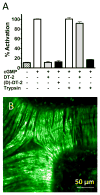
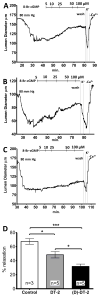
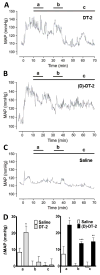
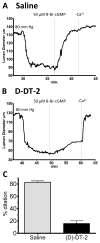
The cGMP-dependent protein kinase type I (PKG I) is an essential regulator of cellular function in blood vessels throughout the body. DT-2, a peptidic inhibitor of PKG, has played a central role in determining the molecular mechanisms of vascular control involving PKG and its signaling partners. Here, we report the development of (d)-amino acid DT-2 derivatives, namely the retro-inverso ri-(d)-DT-2 and the all (d)-amino acid analog, (d)-DT-2. Both peptide analogs were potent PKG Ialpha inhibitors with K(i) values of 5.5 nM (ri-(d)-DT-2) and 0.8 nM ((d)-DT-2) as determined using a hyperbolic mixed-type inhibition model. Also, both analogs were proteolytically stable in vivo, showed elevated selectivity, and displayed enhanced membrane translocation properties. Studies on isolated arteries from the resistance vasculature demonstrated that intraluminally perfused (d)-DT-2 significantly inhibited vasodilation induced by 8-Br-cGMP. Furthermore, in vivo application of (d)-DT-2 established a uniform translocation pattern in the resistance vasculature, with exception of the brain. Thus, (d)-DT-2 caused significant increases in mean arterial blood pressure in unrestrained, awake mice. Further, mesenteric arteries isolated from (d)-DT-2 treated animals showed a markedly reduced dilator response to 8-Br-cGMP in vitro. Our results clearly demonstrate that (d)-DT-2 is a superior inhibitor of PKG Ialpha and its application in vivo leads to sustained inhibition of PKG in vascular smooth muscle cells. The discovery of (d)-DT-2 may help our understanding of how blood vessels constrict and dilate and may also aid the development of new strategies and therapeutic agents targeted to the prevention and treatment of vascular disorders such as hypertension, stroke and coronary artery disease.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures
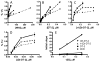


Similar articles
-
Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vasoregulation.Mol Pharmacol. 2004 May;65(5):1111-9. doi: 10.1124/mol.65.5.1111. Mol Pharmacol. 2004. PMID: 15102939
-
Cyclic GMP specifically suppresses Type-Ialpha cGMP-dependent protein kinase expression by ubiquitination.Cell Signal. 2009 Jun;21(6):859-66. doi: 10.1016/j.cellsig.2009.01.014. Epub 2009 Jan 8. Cell Signal. 2009. PMID: 19168131 Free PMC article.
-
Hydrogen peroxide enhances vasodilatation by increasing dimerization of cGMP-dependent protein kinase type Iα.Circ J. 2012;76(7):1792-8. doi: 10.1253/circj.cj-11-1368. Epub 2012 Apr 11. Circ J. 2012. PMID: 22498562
-
Exploring the mechanisms of vascular smooth muscle tone with highly specific, membrane-permeable inhibitors of cyclic GMP-dependent protein kinase Ialpha.Pharmacol Ther. 2002 Feb-Mar;93(2-3):203-15. doi: 10.1016/s0163-7258(02)00189-4. Pharmacol Ther. 2002. PMID: 12191612 Review.
-
Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase.Front Biosci. 2006 Jan 1;11:356-67. doi: 10.2741/1803. Front Biosci. 2006. PMID: 16146737 Review.
Cited by
-
cGMP-Dependent Protein Kinase Inhibitors in Health and Disease.Pharmaceuticals (Basel). 2013 Feb 7;6(2):269-86. doi: 10.3390/ph6020269. Pharmaceuticals (Basel). 2013. PMID: 24275951 Free PMC article.
-
Cancer-selective targeting of the NF-κB survival pathway with GADD45β/MKK7 inhibitors.Cancer Cell. 2014 Oct 13;26(4):495-508. doi: 10.1016/j.ccr.2014.07.027. Cancer Cell. 2014. PMID: 25314077 Free PMC article.
-
A computational approach for designing D-proteins with non-canonical amino acid optimised binding affinity.PLoS One. 2017 Nov 6;12(11):e0187524. doi: 10.1371/journal.pone.0187524. eCollection 2017. PLoS One. 2017. PMID: 29108013 Free PMC article.
-
Synthetic Peptides as cGMP-Independent Activators of cGMP-Dependent Protein Kinase Iα.Chem Biol. 2015 Dec 17;22(12):1653-61. doi: 10.1016/j.chembiol.2015.11.005. Chem Biol. 2015. PMID: 26687482 Free PMC article.
-
Pressure-induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels.Sci Signal. 2016 Oct 11;9(449):ra100. doi: 10.1126/scisignal.aaf6625. Sci Signal. 2016. PMID: 27729550 Free PMC article.
References
-
- Feil R, Feil S, Hofmann F. A heretical view on the role of NO and cGMP in vascular proliferative diseases. Trends Mol Med. 2005;11:71–75. - PubMed
-
- Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. - PubMed
-
- Kim C, Vigil D, Anand G, Taylor SS. Structure and dynamics of PKA signaling proteins. Eur J Cell Biol. 2006;85:651–654. - PubMed
-
- Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci. 2006;11:356–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

