Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats
- PMID: 20018408
- PMCID: PMC2889032
- DOI: 10.1016/j.neurobiolaging.2009.11.022
Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats
Abstract
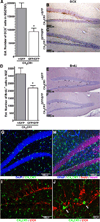
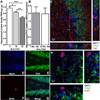
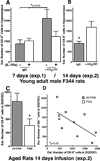
Microglia have neuroprotective capacities, yet chronic activation can promote neurotoxic inflammation. Neuronal fractalkine (FKN), acting on CX(3)CR1, has been shown to suppress excessive microglia activation. We found that disruption in FKN/CX(3)CR1 signaling in young adult rodents decreased survival and proliferation of neural progenitor cells through IL-1β. Aged rats were found to have decreased levels of hippocampal FKN protein; moreover, interruption of CX(3)CR1 function in these animals did not affect neurogenesis. The age-related loss of FKN could be restored by exogenous FKN reversing the age-related decrease in hippocampal neurogenesis. There were no measureable changes in young animals by the addition of exogenous FKN. The results suggest that FKN/CX(3)CR1 signaling has a regulatory role in modulating hippocampal neurogenesis via mechanisms that involve indirect modification of the niche environment. As elevated neuroinflammation is associated with many age-related neurodegenerative diseases, enhancing FKN/CX(3)CR1 interactions could provide an alternative therapeutic approach to slow age-related neurodegeneration.
Published by Elsevier Inc.
Conflict of interest statement
Figures



References
-
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
-
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2008 - PubMed
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

