Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis
- PMID: 20018590
- PMCID: PMC2815873
- DOI: 10.1104/pp.109.150854
Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis
Abstract
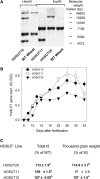
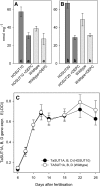
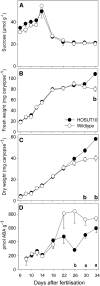
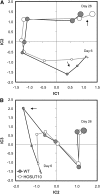
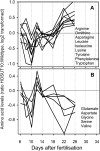
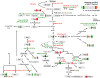
Increasing grain sink strength by improving assimilate uptake capacity could be a promising approach toward getting higher yield. The barley (Hordeum vulgare) sucrose transporter HvSUT1 (SUT) was expressed under control of the endosperm-specific Hordein B1 promoter (HO). Compared with the wild type, transgenic HOSUT grains take up more sucrose (Suc) in vitro, showing that the transgene is functional. Grain Suc levels are not altered, indicating that Suc fluxes are influenced rather than steady-state levels. HOSUT grains have increased percentages of total nitrogen and prolamins, which is reflected in increased levels of phenylalanine, tyrosine, tryptophan, isoleucine, and leucine at late grain development. Transcript profiling indicates specific stimulation of prolamin gene expression at the onset of storage phase. Changes in gene expression and metabolite levels related to carbon metabolism and amino acid biosynthesis suggest deregulated carbon-nitrogen balance, which together indicate carbon sufficiency and relative depletion of nitrogen. Genes, deregulated together with prolamin genes, might represent candidates, which respond positively to assimilate supply and are related to sugar-starch metabolism, cytokinin and brassinosteroid functions, cell proliferation, and sugar/abscisic acid signaling. Genes showing inverse expression patterns represent potential negative regulators. It is concluded that HvSUT1 overexpression increases grain protein content but also deregulates the metabolic status of wheat (Triticum aestivum) grains, accompanied by up-regulated gene expression of positive and negative regulators related to sugar signaling and assimilate supply. In HOSUT grains, alternating stimulation of positive and negative regulators causes oscillatory patterns of gene expression and highlights the capacity and great flexibility to adjust wheat grain storage metabolism in response to metabolic alterations.
Figures

Similar articles
-
Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors.J Exp Bot. 2017 Nov 28;68(20):5511-5525. doi: 10.1093/jxb/erx366. J Exp Bot. 2017. PMID: 29069444 Free PMC article.
-
Down-regulation of the sucrose transporters HvSUT1 and HvSUT2 affects sucrose homeostasis along its delivery path in barley grains.J Exp Bot. 2017 Jul 20;68(16):4595-4612. doi: 10.1093/jxb/erx266. J Exp Bot. 2017. PMID: 28981782 Free PMC article.
-
Barley grains, deficient in cytosolic small subunit of ADP-glucose pyrophosphorylase, reveal coordinate adjustment of C:N metabolism mediated by an overlapping metabolic-hormonal control.Plant J. 2012 Mar;69(6):1077-93. doi: 10.1111/j.1365-313X.2011.04857.x. Epub 2012 Jan 10. Plant J. 2012. PMID: 22098161
-
Seed development and differentiation: a role for metabolic regulation.Plant Biol (Stuttg). 2004 Jul;6(4):375-86. doi: 10.1055/s-2004-817908. Plant Biol (Stuttg). 2004. PMID: 15248120 Review.
-
Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds.J Exp Bot. 2003 Jan;54(382):503-12. doi: 10.1093/jxb/erg051. J Exp Bot. 2003. PMID: 12508061 Review.
Cited by
-
Multi-gene metabolic engineering of tomato plants results in increased fruit yield up to 23%.Sci Rep. 2020 Oct 14;10(1):17219. doi: 10.1038/s41598-020-73709-6. Sci Rep. 2020. PMID: 33057137 Free PMC article.
-
A 'wiring diagram' for sink strength traits impacting wheat yield potential.J Exp Bot. 2023 Jan 1;74(1):40-71. doi: 10.1093/jxb/erac410. J Exp Bot. 2023. PMID: 36334052 Free PMC article. Review.
-
The utility of flow sorting to identify chromosomes carrying a single copy transgene in wheat.Plant Methods. 2016 Apr 25;12:24. doi: 10.1186/s13007-016-0124-8. eCollection 2016. Plant Methods. 2016. PMID: 27118986 Free PMC article.
-
Senescence-induced expression of ZmSUT1 in cotton delays leaf senescence while the seed coat-specific expression increases yield.Plant Cell Rep. 2019 Aug;38(8):991-1000. doi: 10.1007/s00299-019-02421-1. Epub 2019 May 8. Plant Cell Rep. 2019. PMID: 31069498
-
HvPap-1 C1A Protease and HvCPI-2 Cystatin Contribute to Barley Grain Filling and Germination.Plant Physiol. 2016 Apr;170(4):2511-24. doi: 10.1104/pp.15.01944. Epub 2016 Feb 24. Plant Physiol. 2016. PMID: 26912343 Free PMC article.
References
-
- Ambrose MJ, Wang TL, Cook SK, Hedley CL (1987) An analysis of seed development in Pisum sativum L. IV. Cotyledon cell population in vitro and in vivo. J Exp Bot 38 1909–1920
-
- Aoki N, Whitfield P, Hoeren F, Scofield G, Newell K, Patrick J, Offler C, Clarke B, Rahman S, Furbank RT (2002) Three sucrose transporter genes are expressed in the developing grain of hexaploid wheat. Plant Mol Biol 50 453–462 - PubMed
-
- Bagnall N, Wang XD, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. J Plant Physiol 27 1009–1020
-
- Balconi CE, Rizzi E, Manzocchi L, Soave C, Motto M (1991) Analysis of in vivo and in vitro grown endosperms of high and low protein strains of maize. Plant Sci 73 9–18
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

