Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants
- PMID: 20018592
- PMCID: PMC2815900
- DOI: 10.1104/pp.109.147330
Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants
Abstract
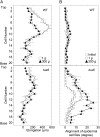
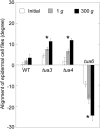
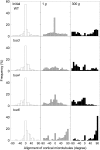
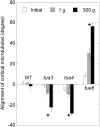
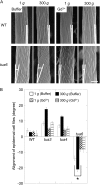
We investigated the roles of cortical microtubules in gravity-induced modifications to the development of stem organs by analyzing morphology and orientation of cortical microtubule arrays in hypocotyls of Arabidopsis (Arabidopsis thaliana) tubulin mutants, tua3(D205N), tua4(S178Delta), and tua6(A281T), cultivated under 1g and hypergravity (300g) conditions. Hypocotyls of tubulin mutants were shorter and thicker than the wild type even at 1g, and hypergravity further suppressed elongation and stimulated expansion. The degree of such changes was clearly smaller in tubulin mutants, in particular in tua6. Hypocotyls of tubulin mutants also showed either left-handed or right-handed helical growth at 1g, and the degree of twisting phenotype was intensified under hypergravity conditions, especially in tua6. Hypergravity induced reorientation of cortical microtubules from transverse to longitudinal directions in epidermal cells of wild-type hypocotyls. In tubulin mutants, especially in tua6, the percentage of cells with longitudinal microtubules was high even at 1g, and it was further increased by hypergravity. The twisting phenotype was most obvious at cells 10 to 12 from the top, where reorientation of cortical microtubules from transverse to longitudinal directions occurred. Moreover, the left-handed helical growth mutants (tua3 and tua4) had right-handed microtubule arrays, whereas the right-handed mutant (tua6) had left-handed arrays. There was a close correlation between the alignment angle of epidermal cell files and the alignment of cortical microtubules. Gadolinium ions, blockers of mechanosensitive ion channels (mechanoreceptors), suppressed the twisting phenotype in tubulin mutants under both 1g and 300 g conditions. Microtubule arrays in tubulin mutants were oriented more transversely by gadolinium treatment, irrespective of gravity conditions. These results support the hypothesis that cortical microtubules play an essential role in maintenance of normal growth phenotype against the gravitational force, and suggest that mechanoreceptors are involved in modifications to morphology and orientation of microtubule arrays by 1g gravity and hypergravity in tubulin mutants.
Figures




Comment in
-
Cortical microtubules are responsible for gravity resistance in plants.Plant Signal Behav. 2010 Jun;5(6):752-4. doi: 10.4161/psb.5.6.11706. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20404495 Free PMC article.
Similar articles
-
Cortical microtubules are responsible for gravity resistance in plants.Plant Signal Behav. 2010 Jun;5(6):752-4. doi: 10.4161/psb.5.6.11706. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20404495 Free PMC article.
-
Involvement of KATANIN1, a microtubule-severing enzyme, in hypergravity-induced modification of growth anisotropy in Arabidopsis hypocotyls.Life Sci Space Res (Amst). 2025 May;45:170-175. doi: 10.1016/j.lssr.2024.11.001. Epub 2024 Nov 6. Life Sci Space Res (Amst). 2025. PMID: 40280639
-
Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2007 May 15;104(20):8544-9. doi: 10.1073/pnas.0701224104. Epub 2007 May 8. Proc Natl Acad Sci U S A. 2007. PMID: 17488810 Free PMC article.
-
Twisted growth and organization of cortical microtubules.J Plant Res. 2007 Jan;120(1):61-70. doi: 10.1007/s10265-006-0039-y. Epub 2006 Oct 24. J Plant Res. 2007. PMID: 17061141 Review.
-
Calcium mobilizations in response to changes in the gravity vector in Arabidopsis seedlings: possible cellular mechanisms.Plant Signal Behav. 2014;9(8):e29099. doi: 10.4161/psb.29099. Plant Signal Behav. 2014. PMID: 25763612 Free PMC article. Review.
Cited by
-
Cortical microtubules are responsible for gravity resistance in plants.Plant Signal Behav. 2010 Jun;5(6):752-4. doi: 10.4161/psb.5.6.11706. Epub 2010 Jun 1. Plant Signal Behav. 2010. PMID: 20404495 Free PMC article.
-
Growth and cortical microtubule dynamics in shoot organs under microgravity and hypergravity conditions.Plant Signal Behav. 2018 Jan 2;13(1):e1422468. doi: 10.1080/15592324.2017.1422468. Epub 2018 Jan 16. Plant Signal Behav. 2018. PMID: 29286875 Free PMC article.
-
Resistance of plants to gravitational force.J Plant Res. 2013 Sep;126(5):589-96. doi: 10.1007/s10265-013-0572-4. Epub 2013 Jun 4. J Plant Res. 2013. PMID: 23732635 Review.
-
An Arabidopsis PTH2 Gene Is Responsible for Gravity Resistance Supporting Plant Growth under Different Gravity Conditions.Life (Basel). 2022 Oct 14;12(10):1603. doi: 10.3390/life12101603. Life (Basel). 2022. PMID: 36295039 Free PMC article.
-
Gravi-Sensitivity of Mosses and Their Gravity-Dependent Ontogenetic Adaptations.Life (Basel). 2022 Nov 4;12(11):1782. doi: 10.3390/life12111782. Life (Basel). 2022. PMID: 36362937 Free PMC article. Review.
References
-
- Abe T, Hashimoto T (2005) Altered microtubule dynamics by expression of modified α-tubulin protein causes right-handed helical growth in transgenic Arabidopsis plants. Plant J 43 191–204 - PubMed
-
- Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215 150–171 - PubMed
-
- Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21 203–222 - PubMed
-
- Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3 83–110 - PubMed
-
- Fasano JM, Massa GD, Gilroy S (2002) Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul 21 71–88 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

