Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus
- PMID: 20018660
- PMCID: PMC2806746
- DOI: 10.1073/pnas.0903158107
Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus
Abstract
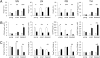
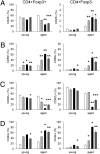
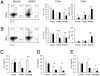
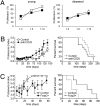
The origins and consequences of a regulatory T cell (Treg) disorder in systemic lupus erythematosus (SLE) are poorly understood. In the (NZBxNZW) F(1) mouse model of lupus, we found that CD4(+)Foxp3(+) Treg failed to maintain a competitive pool size in the peripheral lymphoid organs resulting in a progressive homeostatic imbalance of CD4(+)Foxp3(+) Treg and CD4(+)Foxp3(-) conventional T cells (Tcon). In addition, Treg acquired phenotypic changes that are reminiscent of IL-2 deficiency concomitantly to a progressive decline in IL-2-producing Tcon and an increase in activated, IFN-gamma-producing effector Tcon. Nonetheless, Treg from lupus-prone mice were functionally intact and capable to influence the course of disease. Systemic reduction of IL-2 levels early in disease promoted Tcon hyperactivity, induced the imbalance of Treg and effector Tcon, and strongly accelerated disease progression. In contrast, administration of IL-2 partially restored the balance of Treg and effector Tcon by promoting the homeostatic proliferation of endogenous Treg and impeded the progression of established disease. Thus, an acquired and self-amplifying disruption of the Treg-IL-2 axis contributed essentially to Tcon hyperactivity and the development of murine lupus. The reversibility of this homeostatic Treg disorder provides promising approaches for the treatment of SLE.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. - PubMed
-
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

