Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage
- PMID: 20018662
- PMCID: PMC2806699
- DOI: 10.1073/pnas.0912931107
Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage
Abstract
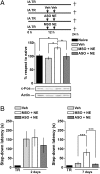
Memory formation is a temporally graded process during which transcription and translation steps are required in the first hours after acquisition. Although persistence is a key characteristic of memory storage, its mechanisms are scarcely characterized. Here, we show that long-lasting but not short-lived inhibitory avoidance long-term memory is associated with a delayed expression of c-Fos in the hippocampus. Importantly, this late wave of c-Fos is necessary for maintenance of inhibitory avoidance long-term storage. Moreover, inhibition of transcription in the dorsal hippocampus 24 h after training hinders persistence but not formation of long-term storage. These findings indicate that a delayed phase of transcription is essential for maintenance of a hippocampus-dependent memory trace. Our results support the hypothesis that recurrent rounds of consolidation-like events take place late after learning in the dorsal hippocampus to maintain memories.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression.Neurobiol Learn Mem. 2012 Oct;98(3):220-7. doi: 10.1016/j.nlm.2012.08.001. Epub 2012 Aug 10. Neurobiol Learn Mem. 2012. PMID: 22906840
-
Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus.Neuron. 2007 Jan 18;53(2):261-77. doi: 10.1016/j.neuron.2006.11.025. Neuron. 2007. PMID: 17224407
-
Increase in c-Fos and Arc protein in retrosplenial cortex after memory-improving lateral hypothalamic electrical stimulation treatment.Neurobiol Learn Mem. 2016 Feb;128:117-24. doi: 10.1016/j.nlm.2015.12.012. Epub 2016 Jan 7. Neurobiol Learn Mem. 2016. PMID: 26774022
-
Persistence of long-term memory storage: new insights into its molecular signatures in the hippocampus and related structures.Neurotox Res. 2010 Nov;18(3-4):377-85. doi: 10.1007/s12640-010-9155-5. Epub 2010 Feb 12. Neurotox Res. 2010. PMID: 20151243 Review.
-
Molecular signatures and mechanisms of long-lasting memory consolidation and storage.Neurobiol Learn Mem. 2013 Nov;106:40-7. doi: 10.1016/j.nlm.2013.06.018. Epub 2013 Jul 3. Neurobiol Learn Mem. 2013. PMID: 23831672 Review.
Cited by
-
Transcranial photobiomodulation prevents PTSD-like comorbidities in rats experiencing underwater trauma.Transl Psychiatry. 2021 May 5;11(1):270. doi: 10.1038/s41398-021-01389-5. Transl Psychiatry. 2021. PMID: 33953158 Free PMC article.
-
The DNA Repair-Associated Protein Gadd45γ Regulates the Temporal Coding of Immediate Early Gene Expression within the Prelimbic Prefrontal Cortex and Is Required for the Consolidation of Associative Fear Memory.J Neurosci. 2019 Feb 6;39(6):970-983. doi: 10.1523/JNEUROSCI.2024-18.2018. Epub 2018 Dec 13. J Neurosci. 2019. PMID: 30545945 Free PMC article.
-
Behavioural tagging: Effect of novelty exploration on plasticity related molecular signatures.Exp Brain Res. 2021 Aug;239(8):2359-2374. doi: 10.1007/s00221-021-06099-4. Epub 2021 Jun 7. Exp Brain Res. 2021. PMID: 34097099
-
Epigenetic regulation of memory formation and maintenance.Learn Mem. 2013 Jan 15;20(2):61-74. doi: 10.1101/lm.026575.112. Learn Mem. 2013. PMID: 23322554 Free PMC article. Review.
-
Biphasic Npas4 expression promotes inhibitory plasticity and suppression of fear memory consolidation in mice.Mol Psychiatry. 2024 Jul;29(7):1929-1940. doi: 10.1038/s41380-024-02454-3. Epub 2024 Feb 13. Mol Psychiatry. 2024. PMID: 38347124 Free PMC article.
References
-
- Squire LR, Barondes SH. Actinomycin-D: Effects on memory at different times after training. Nature. 1970;225:649–650. - PubMed
-
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. - PubMed
-
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

