Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis
- PMID: 20018667
- PMCID: PMC2806762
- DOI: 10.1073/pnas.0911790107
Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis
Abstract
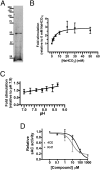
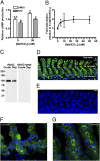
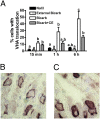
pH homeostasis is essential for life, yet it remains unclear how animals sense their systemic acid/base (A/B) status. Soluble adenylyl cyclase (sAC) is an evolutionary conserved signaling enzyme that produces the second messenger cAMP in response to bicarbonate ions (HCO(3)(-)). We cloned the sAC ortholog from the dogfish, a shark that regulates blood A/B by absorbing and secreting protons (H(+)) and HCO(3)(-) at its gills. Similar to mammalian sAC, dogfish soluble adenylyl cyclase (dfsAC) is activated by HCO(3)(-) and can be inhibited by two structurally and mechanistically distinct small molecule inhibitors. dfsAC is expressed in the gill epithelium, where the subset of base-secreting cells resides. Injection of inhibitors into animals under alkaline stress confirmed that dfsAC is essential for maintaining systemic pH and HCO(3)(-) levels in the whole organism. One of the downstream effects of dfsAC is to promote the insertion of vacuolar proton pumps into the basolateral membrane to absorb H(+) into the blood. sAC orthologs are present throughout metazoans, and mammalian sAC is expressed in A/B regulatory organs, suggesting that systemic A/B sensing via sAC is widespread in the animal kingdom.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Soluble adenylyl cyclase is an acid-base sensor in epithelial base-secreting cells.Am J Physiol Cell Physiol. 2016 Aug 1;311(2):C340-9. doi: 10.1152/ajpcell.00089.2016. Epub 2016 Jun 22. Am J Physiol Cell Physiol. 2016. PMID: 27335168 Free PMC article.
-
Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals.J Exp Biol. 2014 Mar 1;217(Pt 5):663-72. doi: 10.1242/jeb.086157. J Exp Biol. 2014. PMID: 24574382 Free PMC article. Review.
-
Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection.Am J Physiol Cell Physiol. 2011 Feb;300(2):C368-74. doi: 10.1152/ajpcell.00314.2010. Epub 2010 Dec 1. Am J Physiol Cell Physiol. 2011. PMID: 21123735 Free PMC article.
-
Bicarbonate-sensing soluble adenylyl cyclase is present in the cell cytoplasm and nucleus of multiple shark tissues.Physiol Rep. 2017 Jan;5(2):e13090. doi: 10.14814/phy2.13090. Physiol Rep. 2017. PMID: 28108644 Free PMC article.
-
sAC from aquatic organisms as a model to study the evolution of acid/base sensing.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2629-35. doi: 10.1016/j.bbadis.2014.06.021. Epub 2014 Jun 24. Biochim Biophys Acta. 2014. PMID: 24971688 Review.
Cited by
-
Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):607-12. doi: 10.1073/pnas.1413483112. Epub 2014 Dec 29. Proc Natl Acad Sci U S A. 2015. PMID: 25548188 Free PMC article.
-
Acid-base transport by the renal proximal tubule.J Nephrol. 2010 Nov-Dec;23 Suppl 16(0 16):S4-18. J Nephrol. 2010. PMID: 21170887 Free PMC article. Review.
-
Soluble adenylyl cyclase is essential for proper lysosomal acidification.J Gen Physiol. 2016 Oct;148(4):325-39. doi: 10.1085/jgp.201611606. J Gen Physiol. 2016. PMID: 27670898 Free PMC article.
-
Biological responses of sharks to ocean acidification.Biol Lett. 2017 Mar;13(3):20160796. doi: 10.1098/rsbl.2016.0796. Biol Lett. 2017. PMID: 28356408 Free PMC article. Review.
-
Strategies to safely target widely expressed soluble adenylyl cyclase for contraception.Front Pharmacol. 2022 Aug 25;13:953903. doi: 10.3389/fphar.2022.953903. eCollection 2022. Front Pharmacol. 2022. PMID: 36091839 Free PMC article.
References
-
- Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. - PubMed
-
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

