Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells
- PMID: 20018690
- PMCID: PMC2806720
- DOI: 10.1073/pnas.0909541107
Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells
Abstract
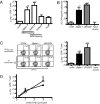
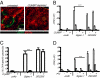
The spleen is the lymphoid organ that induces immune responses toward blood-borne pathogens. Specialized macrophages in the splenic marginal zone are strategically positioned to phagocytose pathogens and cell debris, but are not known to play a role in the activation of T-cell responses. Here we demonstrate that splenic marginal metallophilic macrophages (MMM) are essential for cross-presentation of blood-borne antigens by splenic dendritic cells (DCs). Our data demonstrate that antigens targeted to MMM as well as blood-borne adenoviruses are efficiently captured by MMM and exclusively transferred to splenic CD8(+) DCs for cross-presentation and for the activation of cytotoxic T lymphocytes. Depletion of macrophages in the marginal zone prevents cytotoxic T-lymphocyte activation by CD8(+) DCs after antibody targeting or adenovirus infection. Moreover, we show that tumor antigen targeting to MMM is very effective as antitumor immunotherapy. Our studies point to an important role for splenic MMM in the initial steps of CD8(+) T-cell immunity by capturing and concentrating blood-borne antigens and the transfer to cross-presenting DCs which can be used to design vaccination strategies to induce antitumor cytotoxic T-cell immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
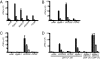


Similar articles
-
Metallophilic marginal zone macrophages cross-prime CD8+ T cell-mediated protective immunity against blood-borne tumors.Immunity. 2025 Apr 8;58(4):843-860.e20. doi: 10.1016/j.immuni.2025.02.027. Epub 2025 Mar 25. Immunity. 2025. PMID: 40139188
-
CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo.J Exp Med. 2000 Dec 18;192(12):1685-96. doi: 10.1084/jem.192.12.1685. J Exp Med. 2000. PMID: 11120766 Free PMC article.
-
Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming.Cell Rep. 2018 Feb 6;22(6):1484-1495. doi: 10.1016/j.celrep.2018.01.021. Cell Rep. 2018. PMID: 29425504
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
-
Antigen Cross-Presentation by Macrophages.Front Immunol. 2020 Jul 8;11:1276. doi: 10.3389/fimmu.2020.01276. eCollection 2020. Front Immunol. 2020. PMID: 32733446 Free PMC article. Review.
Cited by
-
Macrophage heterogeneity in lymphoid tissues.Semin Immunopathol. 2013 Sep;35(5):541-52. doi: 10.1007/s00281-013-0378-4. Epub 2013 Apr 12. Semin Immunopathol. 2013. PMID: 23579230
-
Activation of CD8⁺ T Cell Responses after Melanoma Antigen Targeting to CD169⁺ Antigen Presenting Cells in Mice and Humans.Cancers (Basel). 2019 Feb 5;11(2):183. doi: 10.3390/cancers11020183. Cancers (Basel). 2019. PMID: 30764534 Free PMC article.
-
Tissue-resident macrophages.Nat Immunol. 2013 Oct;14(10):986-95. doi: 10.1038/ni.2705. Epub 2013 Sep 18. Nat Immunol. 2013. PMID: 24048120 Free PMC article. Review.
-
CD169 Expression in Lymph Nodes is Associated with Increased Infiltration of CD8+ T Cells in Tumors: A Systematic Review and Meta-Analysis.J Immunol Res. 2024 Jan 12;2024:8873767. doi: 10.1155/2024/8873767. eCollection 2024. J Immunol Res. 2024. PMID: 38250298 Free PMC article.
-
Targeting Macrophage Polarization for Reinstating Homeostasis following Tissue Damage.Int J Mol Sci. 2024 Jul 2;25(13):7278. doi: 10.3390/ijms25137278. Int J Mol Sci. 2024. PMID: 39000385 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

