Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer
- PMID: 20018721
- PMCID: PMC2806749
- DOI: 10.1073/pnas.0908428107
Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer
Erratum in
-
Correction for Morton et al., Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2204610119. doi: 10.1073/pnas.2204610119. Epub 2022 Apr 26. Proc Natl Acad Sci U S A. 2022. PMID: 35471899 Free PMC article. No abstract available.
Abstract
TP53 mutation occurs in 50-75% of human pancreatic ductal adenocarcinomas (PDAC) following an initiating activating mutation in the KRAS gene. These p53 mutations frequently result in expression of a stable protein, p53(R175H), rather than complete loss of protein expression. In this study we elucidate the functions of mutant p53 (Trp53(R172H)), compared to knockout p53 (Trp53(fl)), in a mouse model of PDAC. First we find that although Kras(G12D) is one of the major oncogenic drivers of PDAC, most Kras(G12D)-expressing pancreatic cells are selectively lost from the tissue, and those that remain form premalignant lesions. Loss, or mutation, of Trp53 allows retention of the Kras(G12D)-expressing cells and drives rapid progression of these premalignant lesions to PDAC. This progression is consistent with failed growth arrest and/or senescence of premalignant lesions, since a mutant of p53, p53(R172P), which can still induce p21 and cell cycle arrest, is resistant to PDAC formation. Second, we find that despite similar kinetics of primary tumor formation, mutant p53(R172H), as compared with genetic loss of p53, specifically promotes metastasis. Moreover, only mutant p53(R172H)-expressing tumor cells exhibit invasive activity in an in vitro assay. Importantly, in human PDAC, p53 accumulation significantly correlates with lymph node metastasis. In summary, by using 'knock-in' mutations of Trp53 we have identified two critical acquired functions of a stably expressed mutant form of p53 that drive PDAC; first, an escape from Kras(G12D)-induced senescence/growth arrest and second, the promotion of metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
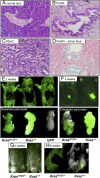
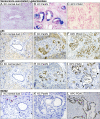
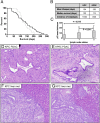

References
-
- Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. - PubMed
-
- Jensen OM, Estève J, Møller H, Renard H. Cancer in the European Community and its member states. Eur J Cancer. 1990;26:1167–1256. - PubMed
-
- Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. - PubMed
-
- Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. - PubMed
-
- Almoguera C, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

