Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling
- PMID: 20018728
- PMCID: PMC2799762
- DOI: 10.1073/pnas.0907464106
Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling
Abstract
Despite long-standing interest, the molecular mechanisms underlying the establishment of anterior-posterior (AP) polarity remain among the unsolved mysteries in metazoans. In the planarians (a family of flatworms), canonical Wnt/beta-catenin signaling is required for posterior specification, as it is in many animals. However, the molecular mechanisms regulating the posterior-specific induction of Wnt genes according to the AP polarity have remained unclear. Here, we demonstrate that Hedgehog (Hh) signaling is responsible for the establishment of AP polarity via its regulation of the transcription of Wnt family genes during planarian regeneration. We found that RNAi gene knockdown of Dugesia japonica patched (Djptc) caused ectopic tail formation in the anterior blastema of body fragments, resulting in bipolar-tails regeneration. In contrast, RNAi of hedgehog (Djhh) and gli (Djgli) caused bipolar-heads regeneration. We show that Patched-mediated Hh signaling was crucial for posterior specification, which is established by regulating the transcription of Wnt genes via downstream Gli activity. Moreover, differentiated cells were responsible for the posterior specification of undifferentiated stem cells through Wnt/beta-catenin signaling. Surprisingly, Djhh was expressed in neural cells all along the ventral nerve cords (along the AP axis), but not in the posterior blastema of body fragments, where the expression of Wnt genes was induced for posteriorization. We therefore propose that Hh signals direct head or tail regeneration according to the AP polarity, which is established by Hh signaling activity along the body's preexisting nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
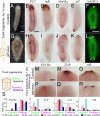
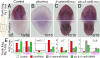

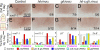
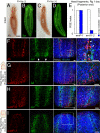

References
-
- Morgan TH. An analysis of the phenomena of organic ‘polarity’. Science. 1904;20:742–748. - PubMed
-
- Morgan TH. The control of heteromorphosis in Planaria maculata. Arch Entw Mech Org. 1904;17:683–695.
-
- Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. - PubMed
-
- McWhinnie MA. The effect of colchicine on reconstitutional development in Dugesia dorotocephala. Bio Bull. 1955;108:54–65.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

