Probing ribosome-nascent chain complexes produced in vivo by NMR spectroscopy
- PMID: 20018739
- PMCID: PMC2799734
- DOI: 10.1073/pnas.0903750106
Probing ribosome-nascent chain complexes produced in vivo by NMR spectroscopy
Abstract
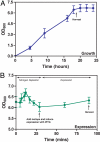
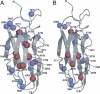
The means by which a polypeptide chain acquires its unique 3-D structure is a fundamental question in biology. During its synthesis on the ribosome, a nascent chain (NC) emerges vectorially and will begin to fold in a cotranslational fashion. The complex environment of the cell, coupled with the gradual emergence of the ribosome-tethered NC during its synthesis, imposes conformational restraints on its folding landscape that differ from those placed on an isolated protein when stimulated to fold following denaturation in solution. To begin to examine cotranslational folding as it would occur within a cell, we produce highly selective, isotopically labeled NCs bound to isotopically silent ribosomes in vivo. We then apply NMR spectroscopy to study, at a residue specific level, the conformation of NCs consisting of different fractional lengths of the polypeptide chain corresponding to a given protein. This combined approach provides a powerful means of generating a series of snapshots of the folding of the NC as it emerges from the ribosome. Application of this strategy to the NMR analysis of the progressive synthesis of an Ig-like domain reveals the existence of a partially folded ribosome-bound species that is likely to represent an intermediate species populated during the cotranslational folding process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

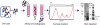


References
-
- Steitz TA. A structural understanding of the dynamic ribosome machine. Nat Rev Mol Cell Biol. 2008;9:242–253. - PubMed
-
- Bashan A, Yonath A. Correlating ribosome function with high-resolution structures. Trends Microbiol. 2008;16:326–335. - PubMed
-
- Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. - PubMed
-
- Mitra K, Frank J. Ribosome dynamics: Insights from atomic structure modeling into cryo-electron microscopy maps. Ann Rev Biophys Biomol Struc. 2006;35:299–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

