Characterization of an electron conduit between bacteria and the extracellular environment
- PMID: 20018742
- PMCID: PMC2799772
- DOI: 10.1073/pnas.0900086106
Characterization of an electron conduit between bacteria and the extracellular environment
Abstract
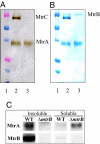
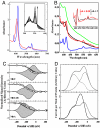
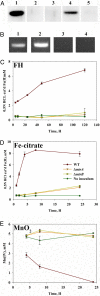
A number of species of Gram-negative bacteria can use insoluble minerals of Fe(III) and Mn(IV) as extracellular respiratory electron acceptors. In some species of Shewanella, deca-heme electron transfer proteins lie at the extracellular face of the outer membrane (OM), where they can interact with insoluble substrates. To reduce extracellular substrates, these redox proteins must be charged by the inner membrane/periplasmic electron transfer system. Here, we present a spectro-potentiometric characterization of a trans-OM icosa-heme complex, MtrCAB, and demonstrate its capacity to move electrons across a lipid bilayer after incorporation into proteoliposomes. We also show that a stable MtrAB subcomplex can assemble in the absence of MtrC; an MtrBC subcomplex is not assembled in the absence of MtrA; and MtrA is only associated to the membrane in cells when MtrB is present. We propose a model for the modular organization of the MtrCAB complex in which MtrC is an extracellular element that mediates electron transfer to extracellular substrates and MtrB is a trans-OM spanning beta-barrel protein that serves as a sheath, within which MtrA and MtrC exchange electrons. We have identified the MtrAB module in a range of bacterial phyla, suggesting that it is widely used in electron exchange with the extracellular environment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Structural modeling of an outer membrane electron conduit from a metal-reducing bacterium suggests electron transfer via periplasmic redox partners.J Biol Chem. 2018 May 25;293(21):8103-8112. doi: 10.1074/jbc.RA118.001850. Epub 2018 Apr 10. J Biol Chem. 2018. PMID: 29636412 Free PMC article.
-
Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6346-51. doi: 10.1073/pnas.1220074110. Epub 2013 Mar 28. Proc Natl Acad Sci U S A. 2013. PMID: 23538304 Free PMC article.
-
Genomic plasticity enables a secondary electron transport pathway in Shewanella oneidensis.Appl Environ Microbiol. 2013 Feb;79(4):1150-9. doi: 10.1128/AEM.03556-12. Epub 2012 Dec 7. Appl Environ Microbiol. 2013. PMID: 23220953 Free PMC article.
-
Mechanisms of Bacterial Extracellular Electron Exchange.Adv Microb Physiol. 2016;68:87-138. doi: 10.1016/bs.ampbs.2016.02.002. Epub 2016 Mar 24. Adv Microb Physiol. 2016. PMID: 27134022 Review.
-
Exploring the biochemistry at the extracellular redox frontier of bacterial mineral Fe(III) respiration.Biochem Soc Trans. 2012 Jun 1;40(3):493-500. doi: 10.1042/BST20120018. Biochem Soc Trans. 2012. PMID: 22616858 Review.
Cited by
-
Iron is not everything: unexpected complex metabolic responses between iron-cycling microorganisms.ISME J. 2020 Nov;14(11):2675-2690. doi: 10.1038/s41396-020-0718-z. Epub 2020 Jul 20. ISME J. 2020. PMID: 32690937 Free PMC article.
-
Effects of Incubation Conditions on Cr(VI) Reduction by c-type Cytochromes in Intact Shewanella oneidensis MR-1 Cells.Front Microbiol. 2016 May 19;7:746. doi: 10.3389/fmicb.2016.00746. eCollection 2016. Front Microbiol. 2016. PMID: 27242759 Free PMC article.
-
Biological Oxidation of Fe(II)-Bearing Smectite by Microaerophilic Iron Oxidizer Sideroxydans lithotrophicus Using Dual Mto and Cyc2 Iron Oxidation Pathways.Environ Sci Technol. 2022 Dec 6;56(23):17443-17453. doi: 10.1021/acs.est.2c05142. Epub 2022 Nov 23. Environ Sci Technol. 2022. PMID: 36417801 Free PMC article.
-
Electromicrobiology: realities, grand challenges, goals and predictions.Microb Biotechnol. 2016 Sep;9(5):595-600. doi: 10.1111/1751-7915.12400. Epub 2016 Aug 10. Microb Biotechnol. 2016. PMID: 27506517 Free PMC article. Review.
-
Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging.Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):16806-10. doi: 10.1073/pnas.1011699107. Epub 2010 Sep 13. Proc Natl Acad Sci U S A. 2010. PMID: 20837546 Free PMC article.
References
-
- Richardson DJ. Bacterial respiration: A flexible process for a changing environment. Microbiology. 2000;146:551–571. - PubMed
-
- Myers CR, Myers JM. Outer membrane cytochromes of Shewanella putrefaciens MR-1: Spectral analysis, and purification of the 83-KDa c-type cytochrome. Biochim Biophys Acta Biomem. 1997;1326:307–318. - PubMed
-
- Myers CR, Nealson KH. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240:1319–1321. - PubMed
-
- Pitts KE, et al. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA. J Biol Chem. 2003;278:27758–27765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

