Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas
- PMID: 20018761
- PMCID: PMC2806716
- DOI: 10.1073/pnas.0912589107
Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas
Abstract
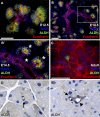
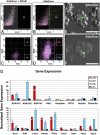
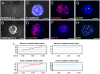
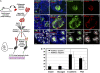
The question of whether dedicated progenitor cells exist in adult vertebrate pancreas remains controversial. Centroacinar cells and terminal duct (CA/TD) cells lie at the junction between peripheral acinar cells and the adjacent ductal epithelium, and are frequently included among cell types proposed as candidate pancreatic progenitors. However these cells have not previously been isolated in a manner that allows formal assessment of their progenitor capacities. We have found that a subset of adult CA/TD cells are characterized by high levels of ALDH1 enzymatic activity, related to high-level expression of both Aldh1a1 and Aldh1a7. This allows their isolation by FACS using a fluorogenic ALDH1 substrate. FACS-isolated CA/TD cells are relatively depleted of transcripts associated with differentiated pancreatic cell types. In contrast, they are markedly enriched for transcripts encoding Sca1, Sdf1, c-Met, Nestin, and Sox9, markers previously associated with progenitor populations in embryonic pancreas and other tissues. FACS-sorted CA/TD cells are uniquely able to form self-renewing "pancreatospheres" in suspension culture, even when plated at clonal density. These spheres display a capacity for spontaneous endocrine and exocrine differentiation, as well as glucose-responsive insulin secretion. In addition, when injected into cultured embryonic dorsal pancreatic buds, these adult cells display a unique capacity to contribute to both the embryonic endocrine and exocrine lineages. Finally, these cells demonstrate dramatic expansion in the setting of chronic epithelial injury. These findings suggest that CA/TD cells are indeed capable of progenitor function and may contribute to the maintenance of tissue homeostasis in adult mouse pancreas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
-
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

