NCLX is an essential component of mitochondrial Na+/Ca2+ exchange
- PMID: 20018762
- PMCID: PMC2806722
- DOI: 10.1073/pnas.0908099107
NCLX is an essential component of mitochondrial Na+/Ca2+ exchange
Abstract
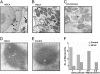
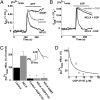
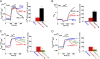
Mitochondrial Ca(2+) efflux is linked to numerous cellular activities and pathophysiological processes. Although it is established that an Na(+)-dependent mechanism mediates mitochondrial Ca(2+) efflux, the molecular identity of this transporter has remained elusive. Here we show that the Na(+)/Ca(2+) exchanger NCLX is enriched in mitochondria, where it is localized to the cristae. Employing Ca(2+) and Na(+) fluorescent imaging, we demonstrate that mitochondrial Na(+)-dependent Ca(2+) efflux is enhanced upon overexpression of NCLX, is reduced by silencing of NCLX expression by siRNA, and is fully rescued by the concomitant expression of heterologous NCLX. NCLX-mediated mitochondrial Ca(2+) transport was inhibited, moreover, by CGP-37157 and exhibited Li(+) dependence, both hallmarks of mitochondrial Na(+)-dependent Ca(2+) efflux. Finally, NCLX-mediated mitochondrial Ca(2+) exchange is blocked in cells expressing a catalytically inactive NCLX mutant. Taken together, our results converge to the conclusion that NCLX is the long-sought mitochondrial Na(+)/Ca(2+) exchanger.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. - PubMed
-
- Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. - PubMed
-
- Cox DA, Matlib MA. A role for the mitochondrial Na(+)-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem. 1993;268:938–947. - PubMed
-
- Szabadkai G, et al. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

