Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah
- PMID: 20018815
- PMCID: PMC2815589
- DOI: 10.1128/JCM.01200-09
Molecular epidemiology of pediatric pneumococcal empyema from 2001 to 2007 in Utah
Abstract
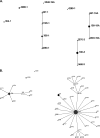
Utah had a high rate of pediatric pneumococcal empyema (PPE) prior to licensure of the pneumococcal conjugate vaccine (PCV-7) in 2000. The majority (62%) of PPE cases was due to nonvaccine serotypes, primarily Streptococcus pneumoniae serotype 1, multilocus sequence type (MLST) 227. PPE in Utah children has increased over the last decade. It is unclear whether the increase was due to serotype replacement or switch. In this study, we describe the incidence and molecular epidemiology of PPE by MLST in Utah children after the licensure of PCV-7. Empyema rates increased from 8.5/100,000 children in the state of Utah in 2001 to 12.5/100,000 children in 2007 (P = 0.006). Ninety-eight percent was due to nonvaccine serotypes (P < 0.001 when compared to the pre-PCV-7 period). PPE was primarily due to serotypes 1, 3, 19A, and 7F, with MLST demonstrating sequence types (ST) that were commonly present in the United States prior to licensure of PCV-7. Serotype switch was not documented. Replacement disease with common ST of serotypes 1,3, 7F, and 19A rather than serotype switch was responsible for the increase in PPE in Utah children.
Figures
References
-
- Advisory Committee on Immunization Practices. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 49:1-35. - PubMed
-
- American Academy of Pediatrics. Committee on Infectious Diseases. 2000. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362-366. - PubMed
-
- Ampofo, K., X. Sheng, K. Korgenski, E. O. Mason, J. Daly, A. T. Pavia, and C. L. Byington. 2009. The changing age and serotype distribution of invasive pneumococcal diseases in the PCV-7 era; implications for new pneumococcal vaccines, abstr. 4315.5. Abstr. 2009 PAS Annu. Meet. Pediatric Academic Societies, Baltimore, MD.
-
- Beall, B., M. C. McEllistrem, R. E. Gertz, Jr., S. Wedel, D. J. Boxrud, A. L. Gonzalez, M. J. Medina, R. Pai, T. A. Thompson, L. H. Harrison, L. McGee, and C. G. Whitney. 2006. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J. Clin. Microbiol. 44:999-1017. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

